

Schering-Plough Political Money
Pushes Claritin Patent Extension and Distorts GAO Report
Click here for the News
Release
Congress Watch
August 2000
Summary
In 1996, the Schering-Plough Corporation began trying to persuade Congress to extend the company’s monopoly patent on its billion-dollar allergy drug Claritin. Since that time, Schering-Plough has dramatically increased its soft money contributions and lobbying expenditures, and hired a "dream team" of lobbyists including Linda Daschle, wife of Senate Minority Leader Tom Daschle (D-S.D.), former House Appropriations Committee Chairman Bob Livingston (R-La.), and Peter Knight, manager of the 1996 Clinton-Gore campaign. The company also made its corporate jet available to presidential candidate Sen. Orrin Hatch (R-Utah), who chairs the Senate committee with jurisdiction over drug patents, in an apparent attempt to help win an extension of Claritin’s monopoly patent.
It’s understandable why Schering-Plough would go to such lengths. The company sold $2.3 billion worth of Claritin last year in the U.S. (1) The patent extension it is seeking could cost consumers $7.3 billion over 10 years because their access to cheaper generic alternatives would be delayed. (2)
For a company making that kind of money, spending $23.9 million since the start of the 1996 election cycle on campaign contributions and lobbying is a small but smart investment. (3) Particularly when it’s buying advice and influence from lobbyists such as Howard Baker, former Republican Senate majority leader, Richard Ben-Veniste, who defended the Clintons during the Senate Whitewater hearings, and Vic Fazio, former chairman of the House Democratic Caucus, as well as Daschle, Livingston and Knight.
This cast has used its considerable legislative cunning on behalf of Schering-Plough. For instance, after the Claritin patent extension bills (H.R. 1598/S. 1172) got stalled in the House and Senate in 1999, a Senate ally of the company tried this year to secretly attach a rider to the fiscal year 2001 Military Construction appropriations bill to achieve Schering-Plough’s goal. Five separate times in 1999 Schering-Plough supplied its corporate jet to presidential candidate Hatch, who chairs the Senate Judiciary Committee, which oversees patents. And at a strategic point in the legislative process last year, the company gave $50,000 in soft money to Sen. John Ashcroft’s (R-Mo.) joint fundraising committee with the National Republican Senatorial Committee. (4) Ashcroft chairs the Senate subcommittee that oversees patents and was considering a presidential bid last year.
Now Schering-Plough is trying to distort the findings of an August 2000 General Accounting Office (GAO) report on patent extensions. (5) The company claims it deserves a monopoly patent for another three years because the Food and Drug Administration (FDA) took too long to approve Claritin. (6) In fact, the GAO found that the company’s flawed application and the FDA’s concern about cancer delayed the Claritin application for justifiable reasons. Don’t be fooled by Schering-Plough – follow the money.
Findings
Lobbying Expenditures
Soft Money Contributions
PAC Candidate Contributions
Contributions to Senate Judiciary Committee Members
High Priced Democratic and Republican Lobbyists Join Claritin's Team
A number of well-connected players signed on to lobby for Schering-Plough in 1999 coming from both sides of the political spectrum. Early in the year the drug giant hired several key Democrats with strong ties to both the Clinton Administration and liberal leaders in Congress, especially on the Judiciary Committees. In June 1999, as the Appropriations bills came into play on the Hill, a Republican consulting firm linked to Senate Majority Leader Trent Lott (R-Miss.) and Appropriations Committee member Sen. Richard Shelby (R-Ala.) joined the team. For good measure, Schering-Plough recently hired former House Appropriations Committee Chairman Bob Livingston. Below are some of Schering-Plough’s lobbyists, their firms and recent earnings. (All data comes from lobby disclosure reports filed with the Clerk of the House and Secretary of the Senate pursuant to the Lobby Disclosure Act of 1995.)
White House, Patent and Trademark Office also Targeted
In addition to Congress, Schering-Plough also needs the Administration to gain its special patent extension for Claritin. Not surprisingly, several key firms reported lobbying the Patent and Trademark Office – the agency to which H.R. 1598/S. 1172 would have given authority to grant the extension – and the White House, which would have to agree to sign the legislation (or not oppose an appropriations rider).
Two firms registered to lobby for the Claritin patent extension list the "White House" as well as Congress on their disclosure forms:
Three firms reported lobbying the Patent and Trademark Office:
The Stakes Are High for
Consumers
H.R. 1598/S.1172 would have allowed, in all likelihood, a three-year patent extension for Claritin and six other "pipeline" drugs (so-called because they were in the FDA approval process at the time the Waxman-Hatch Act was passed in 1984) by creating a slam-dunk review process at the Patent and Trademark Office. The stealth legislation that surfaced this summer (see Chronology section below for details) would have also allowed a three-year patent extension for Claritin – although it would have transferred the review process from the Patent and Trademark Office to a federal Court of Claims. In either case, consumers would pay through the nose. A three-year patent extension for all seven "pipeline" drugs would cost $11 billion over 10 years, with Claritin accounting for two-thirds of that total, according to a University of Minnesota study. (12)
The real impact of the legislation on U.S. health care costs could be much worse. Waiting in the wings to see if the Claritin patent extension bill becomes law are the makers of 20 other blockbuster drugs with combined annual sales of almost $20 billion and patents that expire in the next five years. (13) If some form of a Claritin patent extension gets signed into law, Congress likely would be bombarded by other manufacturers to "tweak" the new patent extension process to fit each of these drugs special circumstances. And if Congress doesn't say "no" to Claritin's special pleading, how will it turn down Prilosec (ulcers), Prozac (depression) and Vasotec (hypertension)?
Chronology of Legislative Events/Soft Money Contributions
A chronology of key legislative events on the Claritin patent extension effort and notable soft money contributions by Schering-Plough is contained in Table 4. It shows the suspicious relation between legislative action and Schering-Plough’s contributions. For instance, the chronology shows Torricelli’s introduction of S. 1172 a day after the company’s $50,000 contribution to the DSCC; the timing of Hatch’s flights on the corporation’s jet relative to his Judiciary Committee hearing on S. 1172; the $50,000 given to the Ashcroft Committee on September 30, 1999, at the height of Senate Judiciary Committee deliberation over S. 1172; and how Sen. Arlen Specter (R-Penn.) tried to attach a Claritin patent extension to the Agriculture Department appropriations bill during the same time period that Schering-Plough gave $200,000 in soft money to Republican campaign committees.
The chronology also tracks the Claritin patent extension bill as it moved in strange and surreptitious ways through the legislative process in late 1999 and the summer of 2000. First, the Senate Judiciary Committee held a hearing on Torricelli’s bill in August 1999. Sen. Hatch did all he could to ram the bill through his Judiciary Committee in October and November in an end-of-the-year rush. But the bill was indefinitely postponed after intense media scrutiny, protests from committee Democrats and signals from several Republican Senators up for re-election that they would not support Hatch’s efforts.
The patent extension resurfaced in June 2000 amid even more controversy. This time it was in legislation written by an anonymous senator. The new proposal was considered even more costly to consumers than S. 1172 because it would automatically extend the Claritin patent while a Federal judge studied the company’s request for an extension. There also was reportedly an attempt to attach the Claritin patent extension to a Military Construction Appropriations bill. That tactic was considered so sneaky that a nonprofit group, The Seniors Coalition, offered a $1,000 reward to anyone who could identify "senator anonymous." Sen. Hatch admitted he was responsible for the text of the bill, but insisted he personally did not try to slip the pro-Claritin language into an appropriations bill. (14)
Eventually, appropriators rejected the idea of adding controversial patent provisions to spending bills. Schering-Plough lobbyist Bob Livingston has reportedly advised the company that any Claritin patent extension must win approval in the Senate Judiciary Committee before it can be considered by all of Congress. (15)
The GAO Report
With the release of the General Accounting Office (GAO) report on August 10, 2000, came signs that Schering-Plough was preparing for another attempt at a patent extension. Schering-Plough’s lobbyists claim the new GAO report supports the company’s argument for extending Claritin's patent. The company contends that the federal Food and Drug Administration needlessly delayed the approval process for Claritin. At one point, Schering-Plough said an FDA reorganization "resulted in inefficiencies and delay when new reviewers rereviewed data that had been accepted by the first reviewers." (16)
While it’s true that the FDA took longer than the average time to approve Claritin (77 months compared to the average of 42.5 months for similarly classified pipeline drugs), it wasn’t because of "inefficiencies" or needless delays by the government. GAO found that the FDA took 77 months to approve Claritin because of concerns about cancer (carcinogenicity) and the company’s flawed application data. More specifically, the FDA focused on tumors found in mice and rats tested with Claritin, and the question of whether the tablet and capsule forms of Claritin are absorbed into the body in equivalent ways. (This is known as "bioequivalence.")
Here are some facts that Public Citizen has gleaned from the GAO report about safety and efficacy issues that concerned the FDA:
In conclusion, it’s important to stress that the FDA’s first responsibility is to the American public and the FDA alone has the sole responsibility for deeming a drug safe and effective. Schering-Plough should not get an extended monopoly for three years – at a cost of $7.3 billion to consumers over 10 years – because it submitted flawed reports.
Table 1: Schering-Plough's Soft Money Contributions to Party Committees, January 1, 1993 – August 31, 2000
|
Democrat |
Republican | ||||
|
Recipient |
Date |
Amount |
Recipient |
Date |
Amount |
| Democratic Congressional Campaign Committee |
5/28/99 |
$25,000 |
Republican National Committee |
8/9//00 |
$100,000 |
| Democratic Senatorial Campaign Committee |
5/26/99 |
$50,000 |
Republican National Committee |
7/20/00 |
$100,000 |
| Democratic Congressional Campaign Committee |
3/25/99 |
$25,000 |
2000 Republican Senate/House Dinner |
5/3/00 |
$20,000 |
| Republican National Committee |
4/28/00 |
$15,000 | |||
| Ashcroft Victory Cmte Non-Federal |
9/30/99 |
$50,000 | |||
| National Republican Senatorial Committee |
9/15/99 |
$50,000 | |||
| Republican National Committee |
9/9/99 |
$1,500 | |||
| National Republican Congressional Committee |
7/16/99 |
$50,000 | |||
| 1999 Republican Senate/House Dinner |
6/3/99 |
$20,000 | |||
| Republican National Committee |
5/5/99 |
$15,000 | |||
| Republican National Committee |
3/31/99 |
$2,000 | |||
| National Republican Congressional Committee |
3/22/99 |
$25,000 | |||
| National Republican Congressional Committee |
3/22/99 |
$75,000 | |||
| National Republican Congressional Committee |
3/10/99 |
$5,000 | |||
| Total (1999 – August 2000) |
$100,000 |
Total (1999 – 2000) |
$528,500 | ||
| Percentage |
16% |
Percentage |
84% | ||
| Democratic National Committee |
11/3/98 |
$1,000 |
National Republican Congressional Committee |
10/9/98 |
$5,000 |
| Democratic Senatorial Campaign Committee |
3/12/98 |
$50,000 |
National Republican Senatorial Committee |
9/30/98 |
$50,000 |
| Democratic Senatorial Campaign Committee |
5/19/97 |
$15,000 |
National Republican Congressional Committee |
9/17/98 |
$100,000 |
| National Republican Congressional Committee |
9/16/98 |
$1,021 | |||
| Republican National Committee |
3/11/98 |
$15,000 | |||
| 1998 Republican Senate/House Dinner |
6/11/97 |
$20,000 | |||
| Republican National Committee |
5/21/97 |
$15,000 | |||
| Republican National Committee |
5/15/97 |
$15,000 | |||
| Total (1997 – 1998) |
$66,000 |
Total (1997 – 1998) |
$221,021 | ||
| Percentage |
23% |
Percentage |
77% | ||
| Democratic Senatorial Campaign Committee |
6/19/96 |
$15,000 |
National Republican Senatorial Committee |
10/25/96 |
$50,000 |
| National Republican Congressional Committee |
9/17/96 |
$100,000 | |||
| Republican National Committee |
8/28/96 |
$15,000 | |||
| National Republican Congressional Committee |
8/11/96 |
$585 | |||
| National Republican Senatorial Committee |
8/7/96 |
$585 | |||
| 1996 Republican Senate/House Dinner |
7/19/96 |
$10,000 | |||
| National Republican Senatorial Committee |
7/15/96 |
$5,000 | |||
| Republican National Committee |
6/28/96 |
$3,000 | |||
| National Republican Senatorial Committee |
6/27/96 |
$2,000 | |||
| 1997 Republican Senate/House Dinner |
6/26/96 |
$10,000 | |||
| Republican National Committee |
6/6/96 |
$100,000 | |||
| National Republican Senatorial Committee |
6/5/96 |
$10,000 | |||
| National Republican Senatorial Committee |
11/6/95 |
$25,000 | |||
| Republican National Committee |
5/24/95 |
$15,000 | |||
| 1995 Republican Senate/House Dinner |
3/15/95 |
$20,000 | |||
| National Republican Congressional Committee |
3/1/95 |
$15,000 | |||
| Total (1995 – 1996) |
$15,000 |
Total (1995 – 1996) |
$381,170 | ||
| Percentage |
4% |
Percentage |
96% | ||
| National Republican Congressional Committee |
11/7/94 |
$25,000 | |||
| Republican National Committee |
11/3/94 |
$5,000 | |||
| Republican National Committee |
10/18/94 |
$10,000 | |||
| National Republican Congressional Committee |
9/30/94 |
$5,000 | |||
| President's Dinner 94 (Repubs) |
5/27/94 |
$20,000 | |||
| Campaign America |
4/19/94 |
$1,000 | |||
| Republican National Committee |
11/17/93 |
$5,000 | |||
| Campaign America |
8/3/93 |
$1,000 | |||
| Campaign America |
5/17/93 |
$5,000 | |||
| President's Dinner 93 (Repubs) |
5/7/93 |
$20,000 | |||
| Total (1993 – 1994) |
$0 |
Total (1993 – 1994) |
$97,000 | ||
| Percentage |
0% |
Percentage |
100% | ||
| Total (1993 – 2000) |
$181,000 |
Total (1993 – 2000) |
$1,227,691 | ||
| Percentage |
13% |
Percentage |
87% |
Note: The National Republican Senate-House Dinner Committee is an annual, joint event to raise funds for the NRCC and NRSC.Source: Figures compiled by Public Citizen from Common Cause (www.commoncause.org) and Center for Responsive Politics (http://www.opensecrets.org/) data.
Table 2: Schering-Plough’s PAC Contributions to Congressional Candidates, January 1, 1995 – June 30, 2000
|
Total |
Democrat |
Republican | |||
|
2000* |
$166,500 |
$64,500 |
39% |
$102,000 |
61% |
|
1998 |
$178,500 |
$46,500 |
26% |
$132,000 |
74% |
|
1996 |
$164,500 |
$33,000 |
20% |
$131,500 |
80% |
|
Total |
$509,500 |
$144,000 |
28% |
$365,500 |
72% |
* Includes all data electronically available from the FEC as of August 3, 2000. Source: Center for Responsive Politics (www.opensecrets.org).
Table 3: Contributions to U.S. Senate Judiciary Committee Members by Schering-Plough’s PAC or Executive Employees
January 1, 1995 – June 30, 2000
|
Senators For |
Party |
Amount |
Position on Claritin Patent Extension |
Congressional Terms on this Committee** |
|
Ashcroft (Mo.) |
R |
$52,000* |
co-sponsor of S. 1172 |
105th-106th |
|
Torricelli (N.J.) |
D |
$34,800 |
lead sponsor of S. 1172 |
106th |
|
Hatch (Utah) |
R |
$20,000 |
publicly endorsed S. 1172 |
104th -106th |
|
Thompson (Tenn.) |
R |
$11,514 |
sponsor of 1997 attempt |
104th -105th |
|
Sessions (Ala.) |
R |
$10,448 |
co-sponsor of S. 1172 |
105th -106th |
|
Specter (Penn.) |
R |
$6,000 |
sponsor of 1996 attempt |
104th-106th |
|
Thurmond (S.C.) |
R |
$0 |
co-sponsor of S. 1172 |
104th-106th |
|
Total |
$134,762 |
|||
|
Senators Against or Undeclared |
| |||
|
DeWine (Ohio) |
R |
$2,000 |
not a co-sponsor |
104th-106th |
|
Abraham (Mich.) |
R |
$1,000 |
not a co-sponsor |
104th-106th |
|
Kyl (Ariz.) |
R |
$1,000 |
not a co-sponsor |
104th-106th |
|
Grassley (Iowa) |
R |
$1,000 |
not a co-sponsor |
104th-106th |
|
Simpson (Wyo.) |
R |
$1,000 |
not a co-sponsor |
104th |
|
Smith (N.H.) |
R |
$300 |
not a co-sponsor |
106th |
|
Biden (Del.) |
D |
$0 |
not a co-sponsor |
105th -106th |
|
Brown (Colo.) |
R |
$0 |
not a co-sponsor |
104th |
|
Durbin (Ill.) |
D |
$0 |
not a co-sponsor |
105th |
|
Feingold (Wis.) |
D |
$0 |
not a co-sponsor |
104th-106th |
|
Feinstein (Calif.) |
D |
$0 |
not a co-sponsor |
104th-106th |
|
Heflin (Ala.) |
D |
$0 |
not a co-sponsor |
104th |
|
Kennedy (Mass.) |
D |
$0 |
not a co-sponsor |
104th-106th |
|
Kohl (Wis.) |
D |
$0 |
not a co-sponsor |
104th-106th |
|
Leahy (Vt.) |
D |
$0 |
not a co-sponsor |
104th-106th |
|
Schumer (N.Y.) |
D |
$0 |
not a co-sponsor |
106th |
|
Simon (Ill.) |
D |
$0 |
not a co-sponsor |
104th |
|
Total |
$6,300 |
| ||
Source: Figures compiled by Public Citizen from Center for Responsive Politics (http://www.opensecrets.org/) data.
*Includes a $50,000 soft money contribution to the Ashcroft Victory Committee, a joint fund-raising account set up by Ashcroft and the National Republican Senatorial Committee.
** Refers only to Congressional terms that coincide with Schering-Plough’s patent extension effort. In other words, only the Congressional terms since 1995.
Figure 1: Total Contributions to Senate Judiciary Committee Members Who Have Supported Claritin Patent Legislation vs. Those Committee Members Who Are Opposed Or Undeclared
(January 1, 1995 – June 30, 2000)
Source: Compiled by Public Citizen using Center for Responsive Politics (http://www.opensecrets.org/) data.
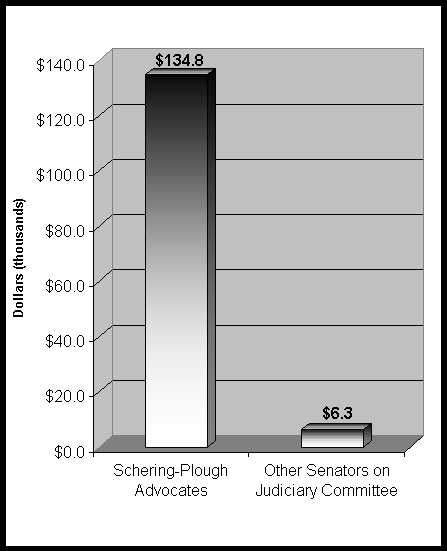
Figure 2: Schering-Plough Lobbying Expenditures (1996 – 1999)*
Source: Lobby disclosure reports filed with the Clerk of the House and Secretary of the Senate pursuant to the Lobby Disclosure Act of 1995.
*Schering-Plough reported spending $3.9 million on lobbying in the first half of of the year 2000.Table 4: Legislative Events on Claritin Patent Extension and Notable Soft Money Contributions and
Expenditures by Schering-Plough as of August 10, 2000
|
Legislative Activity |
Contributions and Expenditures |
| Spring 1996
Schering-Plough’s appeal to Congress began in the spring of 1996, not long after Searle & Co. received a patent extension for its arthritis drug. Summer 1996 Sen. Arlen Specter (R-Penn.) tries unsuccessfully to attach two-year Claritin patent extension to the Agriculture Department appropriations bill.
Early 1997 Schering-Plough begins to court Senators to support legislation to create a stacked-deck patent review process for Claritin and six other pipeline drugs.
May 1998 Rep. Ed Bryant’s (R-Tenn.) effort to insert Claritin patent extension proposal into unrelated patent legislation in House Judiciary Subcommittee on Courts and Intellectual Property fails.
October 1998 Sen. Frank Lautenberg (D-N.J.) tries to attach Claritin’s pipeline drug proposal to omnibus appropriations bill.
April 28, 1999 Rep. Bryant introduces Claritin’s pipeline drug patent extension proposal (H.R. 1598).
May 27, 1999 Sen. Torricelli introduces Claritin’s pipeline drug patent extension proposal (S. 1172).
July 1, 1999 House Judiciary Subcommittee on Courts and Intellectual Property holds hearing on H.R. 1598.
August 4, 1999 Sen. Orrin Hatch (R-Utah), chair of the Judiciary Committee, holds hearing on S. 1172.
November 4, 1999 Sen. Torricelli raises prospect of amending S. 1172 into unrelated legislation during Senate Judiciary Committee mark up. November 10, 1999 Sen. Hatch schedules Senate Judiciary Committee mark-up of S. 1172. November 17, 1999 Consideration of S. 1172 indefinitely postponed by Senate Judiciary Committee. Senators cite Democratic opposition and hectic end-of-year schedule as reasons for inaction.
May 26, 2000 Sen. Judd Gregg (R-N.H.) slips patent extension for his alma mater Columbia University into Agriculture Department appropriations bill.
June 16, 2000 ABC News reports that an anonymous senator tried to attach a Claritin patent extension amendment to a Military Construction appropriations bill during a closed-door House-Senate conference.
June 21, 2000 The Seniors Coalition offers a $1,000 reward to anyone who can identify "senator anonymous." June 25, 2000 Sen. Hatch says, "I’m ‘Mr. Anonymous,’" but qualifies his confession. While his staff did author the language, Hatch says he did not try to sneak it into the appropriations bill. June 26, 2000 ABC News says other leading suspects – Senators Gregg, Torricelli and Conrad Burns (R-Mont.) – deny that they tried to sneak Claritin’s patent extension into the military appropriations bill. July 2000 Appropriators reject Claritin proposal because they don’t want their bills cluttered with controversial provisions.
Roll Call says Livingston has advised Schering-Plough that patent extension can’t be slipped into an appropriations bill; the Senate Judiciary Committee must first pass it. |
1995-1996
Schering-Plough gives a total of only $15,000 in soft money to Democratic Party committees while giving $381,170 to Republican Party committees. June 6, 1996 Schering-Plough contributes $100,000 in soft money to the RNC. September 17, 1996 $100,000 in soft money to the NRCC. October 25, 1996 $50,000 in soft money to the NRSC. 1997-1998 Schering-Plough gives a total of $66,000 in soft money to Democratic Party committees and $220,000 to Republican Party committees. March 12, 1998 $50,000 in soft money to the DSCC (first-time ever contribution), which was vice-chaired by Sen. Robert Torricelli (D-N.J.).
September 17, 1998 $100,000 in soft money to the NRCC. September 30, 1998 $50,000 in soft money to the NRSC.
1999- July 2000 Schering-Plough gives a total of $100,000 in soft money to Democratic Party committees and $428,500 to Republican Party committees. March 22, 1999 $100,000 in soft money to the NRCC. March 25, 1999 $25,000 in soft money to the DCCC.
May 26, 1999 $50,000 in soft money to the DSCC, which is chaired by Sen. Torricelli.
May 28, 1999 Another $25,000 to the DCCC brings Schering-Plough’s soft money donations to the Democrats to $100,000 for 1999. In just the first nine months of 1999, Claritin’s makers have given more than the total it gave to Democrats in the preceding four years.
July 22 - August 21, 1999 Sen. Hatch’s presidential campaign committee makes five reimburse-ments totaling $18,961.50 to Schering-Plough for use of its corporate jet. At least one trip was to Iowa where a Republican Party straw poll for the presidency was held on August 14. September 15, 1999 $50,000 in soft money to the NRSC. September 30, 1999 $50,000 to Ashcroft Victory Committee, a nonfederal joint fundraising committee set up with the NRSC. Sen. John Ashcroft (R-Mo.) chairs a key Judiciary subcommittee with drug patent oversight.
February 14, 2000 Schering-Plough reports lobbying expenses of $7 million in second half of 1999, bringing year’s total lobbying bill to $9.2 million – more than double 1998 expenditures. April 28, 2000 $15,000 in soft money to Republican National Committee.
May-June 2000 Schering-Plough’s PAC contributes $31,000 in "hard" money to candidates, including $5,000 each to H.R. 1598 co-sponsors Rep. Jim Gibbons (R-Nev.) and Rep. Jim McDermott (D-Wash.). Company also gives $5,000 each to U.S. Senate candidate Rick Lazio (R-N.Y.) and Rep. Bill Thomas (R-Calif.), sponsor of pro-drug company bill that would offer senior citizens prescription drug coverage through private insurers instead of Medicare. June 2000 Former House Appropriations Committee Chairman Bob Livingston hired by Schering-Plough to lobby for patent extension.
July 20, 2000 $100,000 in soft money to RNC. |
Endnotes
Click Here for the News Release Associated With This Report
Back to the Prescription Drugs Home Page
Back to the Health Care Home Page
Back to the Health, Safety and Environment Home Page
Back to the Congress Watch Home Page