
Prescription Drug Price Discrimination
in the 5th Congressional District in Florida
Drug Manufacturer Prices Are Higher for
Humans than for Animals
______________________________________________________________________________
Prepared for Rep. Karen L. Thurman
Minority Staff
Special Investigations Division
Committee on Government Reform
U.S. House of Representatives
March 16, 2000
Table of
Contents
Executive
Summary
I. The
Impact of High Drug Prices
II.
Objective of the Study
III.
Methodology
A.
Selection of Drugs
1.
Popular Drugs
2.
Directly Comparable Drugs
B.
Determination of Manufacturer-Level Prices for Humans
C.
Determination of Manufacturer-Level Prices for Animals
D.
Comparison of Manufacturer-Level Prices
E.
Evaluation of Impacts on Uninsured Consumers
IV.
Results of Price Comparisons
A.
Manufacturer Prices Are Over Twice as High for Humans as for Animals
1.
Price Differentials for Popular Prescription Drugs
2.
Price Differentials for Directly Comparable Prescription Drugs
B.
Price Differentials Can Be Substantial in Dollar Terms
C.
Retail-Level Price Differentials Between Human and Animal Drugs Can Be
Significant
V. Drug Prices for Uninsured Consumers in the
5th Congressional District in
Florida Could Be Significantly Reduced by
Preventing Price Discrimination
VI. Quality
Differences and Research Costs Do Not Appear to Explain The Price
Differentials
A.
Drug Quality and Production Cost
B.
Research and Development Costs
VII.
Conclusion
Appendices
EXECUTIVE
SUMMARY
This report on prescription drug pricing was
prepared at the request of Karen L. Thurman,
who represents the 5th Congressional
District in Florida. Rep.
Thurman asked the minority staff of the
Committee on Government Reform to investigate two important questions relating
to the pricing of human and animal drugs: (1) whether drug manufacturers who
sell the same drug for both human use and animal use charge different prices for
human use than for animal use; and (2) if the manufacturers are charging
different prices, what impacts these pricing practices have on drug costs for
consumers in Rep. Thurman's district.
The report finds that drug manufacturers are
engaging in substantial price discrimination, charging low prices for drugs when
they are used by animals and high prices for the same drugs when they are used
by seniors and other consumers who pay for their own drugs. The report also
finds that drug prices for uninsured consumers in the 5th Congressional District in Florida could be significantly reduced if drug manufacturers
eliminated this price discrimination.
This report investigates drug pricing at the
manufacturer level. Its focus is the prices that drug manufacturers charge
wholesalers of human and animal drugs rather than the prices that wholesalers
charge pharmacists and veterinarians or the prices that pharmacists and
veterinarians charge individual consumers. Because most animal drugs are
purchased from veterinarians (who both prescribe and dispense medications) and
not from pharmacists (who only dispense medications), the human and animal drug
markets have substantially different characteristics at the retail level.
Although retail-level price comparisons are analyzed in one part of this report,
differences between the human and animal drug markets at the retail level make
the two markets less comparable at the retail level than at the manufacturer
level.
A. Methodology
Many drugs that are approved and sold for human
use are also approved and sold for animal use. Under the applicable FDA
regulations, both the human and animal versions of the drugs must meet the same
standards for quality and purity. This report investigates the pricing of two
groups of drugs that are approved for both human and animal use. The animals
that use these drugs include cattle, horses, dogs, and cats.
First, the report analyzes the pricing of
popular brand name prescription drugs that are used by both humans and animals.
These are drugs that meet the following criteria: (1) they are among the 200
most popular prescription drugs used by humans during 1998; (2) they are
approved by FDA for both human and animal use; (3) they are dispensed to humans
and animals for consumption through the same dosage route; and (4) they are
commonly available by out-patient prescription. Eight drugs meet these
criteria.
Among these eight popular drugs, some are sold
in a different dosage to humans than animals, and several are made by different
manufacturers for the human and animal markets. To determine if these factors
affected the findings, the report analyzes a second group of drugs that are
directly comparable in their human and animal versions. The drugs in the second
group are brand name drugs that meet the following criteria: (1) they are
approved by FDA for both human and animal use; (2) they are dispensed to humans
and animals in the same dosage for consumption through the same dosage route;
(3) they are manufactured for human and animal use by the same (or related)
companies; and (4) they are commonly available for human use by out-patient
prescription. Eight drugs also meet these criteria. Two of these eight drugs -
Lodine and Vasotec - are also in the first group of popular drugs.
B. Findings
Drug manufacturers charge substantially
more for popular drugs when the drugs are used by humans than when the drugs are
used by animals. Eight brand name drugs among the top 200 are taken
through the same dosage route by both humans and animals and are commonly
obtained via out-patient prescription. For these eight popular drugs, drug
manufacturers charge an average of 106% to 151% more when the drug is intended
for human use than when the drug is intended for animal use. This means that the
average manufacturer-level price for human use is more than twice as much as the
manufacturer-level price for animal use.
Drug manufacturers charge substantially
more for directly comparable drugs when the drugs are used by humans than when
the drugs are used by animals. Similar results were obtained when the
report examined the pricing of the eight brand name drugs that are made for the
human and animal markets in the same dosages by the same (or related) companies.
For this group of eight directly comparable drugs, manufacturers charge an
average of 131% more when the drug is intended for human use than when the drug
is intended for animal use. This price differential is similar to the price
differential observed for the eight popular drugs.
In dollar terms, the price differential
can be substantial. The drug with the largest price differential in
dollar terms is Lodine, a popular arthritis medicine used by both humans and
dogs. American Home Products charges $108.90 for a one-month supply of Lodine
when the drug is to be used by humans, but only $37.80 when the drug is to be
used by dogs. Another drug with a large price difference is Vasotec, a high
blood pressure medication that was the 14th most frequently prescribed human
drug in the United States in 1998. Merck charges $78.55 for a one-month supply
when the drug is to be used by humans, but only $51.30 when the drug is to be
used by dogs - an annual difference of over $325.
Drug prices for uninsured consumers in the
5th Congressional District in Florida could
be significantly reduced by preventing price discrimination. Based on a survey of pharmacists in Rep.
Thurman's district, the report calculates an
upper-bound estimate of the potential savings to individuals who must pay for
their own drugs, such as senior citizens without prescription drug coverage,
from eliminating price discrimination. If the eight popular drugs analyzed in
this report were sold by manufacturers at the same prices for human use that
they are currently sold for animal use, the prices of these drugs for uninsured
consumers in the 5th Congressional District
in Florida could be reduced by an average of
24% to 37%.
The price differentials cannot be
adequately explained by quality differences or research costs. The price
differentials observed in this report appear to be directly attributable to the
deliberate pricing strategies of the drug manufacturers. The report analyzes
whether differences in drug quality, drug production costs, or research and
development expenses are likely causes of the price differentials. None of these
factors appears to account adequately for the discriminatory pricing practices
found in the report.
I. THE IMPACT OF HIGH
DRUG PRICES
Prescription drug costs are rising and causing
increasing hardship for consumers who must pay for their own drugs. In 1990,
prescription drug expenditures in the United States were $37.7 billion
dollars.(1)
By 1998, prescription drug expenditures had more than doubled to $93.4 billion,
due to a combination of price increases and increased utilization.(2)
From 1992 to 1997, prescription drug expenditures rose by more than 11%
annually.(3)
Reducing the costs of prescription drugs has become a national issue, with
numerous proposals pending in Congress.
Much of the discussion of this issue has focused
on the plight of senior citizens, who make up 12% of the population, but use
one-third of all prescription drugs.(4)
Although the elderly have the greatest need for prescription drugs, they often
have the most inadequate insurance coverage for drugs. With the exception of
drugs administered during inpatient hospital stays, Medicare generally does not
cover prescription drugs. According to a recent analysis by the National
Economic Council, approximately 75% of Medicare beneficiaries lack dependable,
private-sector prescription drug coverage.(5)
A recent study by federal researchers found that 32% of Medicare recipients -
over 10 million seniors - do not have any insurance coverage for prescription
drugs.(6)
Because of the high costs of prescription drugs,
uninsured consumers in general - and seniors in particular - face enormous
hardships paying for the medications they need. A recent study found that
prescription drug expenditures are the single largest out-of-pocket health care
cost for senior citizens on Medicare.(7)
Approximately 4.5 million senior citizens spend over $1,000 annually on
prescription drugs, a significant burden for individuals living on a fixed
income.(8)
Indeed, one study found that more than one in eight seniors are forced to choose
between buying food and paying for prescription drugs.(9)
As a consequence of high drug prices, millions of senior citizens and other
uninsured consumers must go without necessary medications, skip doses, or take
less than their prescribed doses, thereby endangering their health.(10)
II. OBJECTIVE OF THE
STUDY
One of the root causes of high drug prices is
manufacturer price discrimination. In 1998, the Congressional Budget Office
(CBO) conducted a detailed examination of drug pricing. CBO found that drug
manufacturers engage in price discrimination that forces uninsured consumers to
pay the highest prices for drugs. According to CBO:
Different buyers pay different prices for
brand-name prescription drugs. . . . In today's market for outpatient drugs,
purchasers that have no insurance coverage for drugs . . . pay the highest
prices for brand name drugs.(11)
In March 1999, the Federal Trade Commission
(FTC) released a comprehensive analysis of prescription drug pricing that
reached a similar conclusion. As in the CBO study, the FTC study found that drug
manufacturers engage in price discrimination. According to the FTC:
A notable example of differential pricing is the
so-called "two tiered pricing structure" under which pharmaceutical companies
set lower prices to large buyers like hospitals, HMOs, and PBMs, and charge
higher prices to other buyers that include the uninsured and independent and
chain retail pharmacies.(12)
While these and other independent experts have
concluded that drug manufacturers engage in price discrimination, there have
been few analyses that quantify the extent of this discrimination. The first two
reports to quantify the extent of this price discrimination in
Florida's 5th congressional district were released by Rep.
Thurman. These reports showed that (1)
uninsured senior citizens in the 5th
Congressional District in Florida pay over
twice as much for prescription drugs as favored customers like HMOs and the
federal government,(13)
and that (2) uninsured senior citizens in Florida's 5th congressional district also pay far higher prices than
do purchasers in Canada and Mexico.(14)
This study seeks to quantify the extent of price
discrimination in a third way. It compares the prices that drug manufacturers
charge for drugs used by humans with the prices that the manufacturers charge
for the same drugs when used by animals. It is the first study to estimate the
effect of this type of price discrimination on drug costs for uninsured
consumers in Rep. Thurman's congressional
district in Florida, such as senior citizens
who pay for their own drugs.
III.
METHODOLOGY
A. Selection of
Drugs
Approximately 37,000 prescription drug products
in the United States are approved for human consumption.(15)
These 37,000 drugs contain approximately 2,500 different active
ingredients.(16)
In 1998, drug manufacturers sold almost $100 billion worth of these
pharmaceuticals for use in the United States.(17)
The animal drug market is smaller, but is
growing rapidly. Over 1,500 drug products in the United States are approved for
animal consumption.(18)
These drugs contain approximately 400 different active ingredients.(19)
Drug manufacturers are continuing to seek approval for new drugs for the animal
market, particularly for companion animals.(20)
In 1998, drug manufacturers sold approximately $3.1 billion worth of
pharmaceuticals for animal use.(21)
There is a substantial overlap between drugs
approved for human and animal use. Of the approximately 400 active ingredients
found in animal drugs, approximately 80 are approved by the Food and Drug
Administration for use by both humans and animals. In total, about 400 animal
drugs contain active ingredients that are also found in human drugs. In
many other cases, veterinarians prescribe products that are approved for human
use for use in animals.(22)
This study analyzed two sets of brand name
drugs. The first set of drugs is comprised of popular brand name drugs that are
manufactured for both human and animal use. The second set of drugs is comprised
of brand name drugs that are directly comparable in their human and animal
versions.(23)
1. Popular
Drugs
The first set of drugs
analyzed in this report are brand name drugs that meet the following criteria:
(1) the drug is among the 200 most popular drugs used by humans during 1998; (2)
the drug is approved by FDA for both human and animal use; (3) the drug is
dispensed to humans and animals for consumption through the same dosage
route;(24)
and (4) the drug is commonly available for human use by out-patient
prescription. Drug popularity was determined based on the 1998 listings by
Pharmacy Times of (1) the top 200 drugs ranked by dollar sales and (2)
the top 200 drugs ranked by number of prescriptions filled.(25)
Any drug on either list was considered to be one of the top 200 drugs used by
humans in 1998.
The following eight drugs meet these four
criteria:
- Amoxil, which is manufactured by SmithKline
Beecham for sale to the human market and by A.H. Robbins for sale to the
animal market under the brand name Robamox. Amoxil is an antibiotic and was
the 45th most frequently prescribed human drug in the United States in 1998.
The product is approved on the animal market as an antibiotic to treat dogs
and cats.
- Augmentin, which is manufactured by
SmithKline Beecham for sale to the human market and by Pfizer for sale to the
animal market under the brand name Clavamox. Augmentin is an antibiotic and
was the 12th best selling human drug in dollar sales in the United States in
1998. The product is approved on the animal market as an antibiotic to treat
dogs and cats.
- Bactroban, which is manufactured by
SmithKline Beecham for sale to the human market and by Pfizer for sale to the
animal market under the brand name Bactoderm. Bactroban is a topical
antibiotic and was the 121st most frequently prescribed human drug in the
United States in 1998. The product is approved on the animal market to treat
infections in dogs.
- Lanoxin, which is manufactured by Glaxo
Wellcome for sale to the human market and by Evsco for sale to the animal
market under the brand name Cardoxin LS. Lanoxin is used to treat heart
failure in humans and was the 9th most frequently prescribed human drug in the
United States in 1998. The product is approved on the animal market to treat
heart failure in dogs.
- Lasix, which is manufactured by
Hoechst-Marion Roussel. Lasix is used to treat high blood pressure and heart
problems in humans and was the 160th most frequently prescribed human drug in
the United States in 1998. The product is approved on the animal market to
treat edema in cattle, dogs, and cats.
- Lodine, which is manufactured by
Wyeth-Ayerst, a subsidiary of American Home Products, for sale to the human
market and by Fort Dodge, another subsidiary of American Home Products, for
sale to the animal market under the brand name Etogesic. Lodine is used to
treat arthritis in humans and was the 143rd best selling human drug in dollar
sales in the United States in 1998. The product is approved on the animal
market to treat arthritis in dogs.
- Stadol, which is manufactured by
Bristol-Myers Squibb for sale to the human market and by a subsidiary of
American Home Products for sale to the animal market under the brand names
Torbutrol and Torbugesic. Stadol is used as a pain reliever in humans and was
the 195th best selling human drug in dollar sales in the United States in
1998. The product is approved on the animal market as a pain reliever in dogs
and horses.
- Vasotec, which is manufactured by Merck for
sale to the human market and by Merial, a subsidiary, for sale to the animal
market under the brand name Enacard. Vasotec is used to treat high blood
pressure in humans and was the 14th most frequently prescribed human drug in
the United States in 1998. The product is approved on the animal market to
treat heart failure in dogs.
2. Directly
Comparable Drugs
Among the eight popular drugs, three (Lasix,
Amoxil, and Bactroban) are manufactured in different dosages for the two
markets.(26)
In addition, five drugs (Amoxil, Augment, Bactroban, Lanoxin, and Stadol) are
made by different manufacturers for the two markets. In order to determine if
these factors account for the observed price differentials, a second group of
brand name drugs that are directly comparable in their human and animal versions
was also analyzed in this report. These drugs were chosen based on the following
criteria: (1) the drug is approved by FDA for use in both humans and animals;
(2) the drug is dispensed to humans and animals in the same dosage for
consumption through the same dosage route; (3) the drug is manufactured by the
same company (or by affiliates, subsidiaries, or partners of the same company)
for both the human and animal markets; and (4) the drug is commonly available
for human use by out-patient prescription.
Eight drugs meet these four criteria. Two of
these eight - Lodine and Vasotec - are also included in the list of popular
drugs. The six additional drugs that meet the four criteria are:
- Cleocin, which is manufactured by Pharmacia
and Upjohn and sold in the animal market under the brand name Antirobe.
Cleocin is an antibiotic used to treat bacterial infections in humans. The
product is approved on the animal market to treat bacterial infections in
dogs.
- Fulvicin U/F, which is manufactured by
Schering Plough. Fulvicin U/F is antifungal agent used to treat infections of
the skin, hair, and scalp in humans. The product is approved on the animal
market as an antifungal medication in dogs and cats.
- Medrol, which is manufactured by Pharmacia
and Upjohn. Medrol is used to treat arthritis, allergies, and asthma in
humans. The product is approved on the animal market as an anti-inflammatory
medication in dogs and cats.
- Robaxin, which is manufactured by A.H.
Robins. Robaxin is used an anti-inflammatory pain reliever in humans. The
product is approved on the animal market for use as a pain reliever in dogs
and cats.
- Robinul, which is manufactured by A.H.
Robins. Robinul is used in treatment of peptic ulcers in humans. The product
is approved on the animal market as a pre-anaesthetic agent for use in dogs
and cats.
- Winstrol, which is manufactured by
Sanofi.(27)
Winstrol is used to treat end stage renal disease, anemia, angioedema, and
chronic weight loss following major surgery in humans. The product is approved
on the animal market to treat weight loss, debility, and other symptoms
associated with old age or trauma in dogs and cats.
B. Determination of Manufacturer-Level Prices for
Humans
This report calculates the prices that drug
manufacturers charge for human drugs based on the Wholesale Acquisition Cost
(WAC) that human drug wholesalers pay to acquire drugs for sale to pharmacists.
WAC prices represent the average price that drug manufacturers charge human drug
wholesalers for products that are intended for resale to pharmacists. WAC prices
do not include rebates or other forms of discounts that favored customers like
HMOs often receive.
The prices paid by pharmacists for drugs are
slightly higher than the prices charged by the drug manufacturers to
wholesalers, because the prices paid by pharmacists incorporate a markup by the
drug wholesaler. Typical wholesale markups are small, about 2% to 4% above
WAC.(28)
C.
Determination of Manufacturer-Level Prices for Animals
To determine the prices that drug manufacturers
charge for drugs used by animals, the minority staff obtained the prices that
animal drug wholesalers sell the drugs investigated in this report to
veterinarians. Staff obtained these prices for five major animal drug
wholesalers and determined an average price that animal drug wholesalers charge
veterinarians for each of the drugs. To determine the manufacturer-level price
for the drugs, these average wholesale-level prices were adjusted to eliminate
the effect of the markup charged by animal drug wholesalers.(29)
Two drugs of the drugs examined in this report
are not sold to veterinarians through animal drug wholesalers, but are purchased
by veterinarians directly from the drug manufacturer. The prices that the
manufacturer charges for these two drugs were obtained by the minority staff. No
adjustment was made to account for the effect of a wholesale markup since these
prices were already manufacturer-level prices.
D. Comparison of
Manufacturer-Level Prices
Drug selection for this analysis was based on
criteria that were designed to minimize the differences, if any, between the
human and animal versions of drugs being compared. All drugs in this analysis
are sold in the same dosage route to both the human and animal markets. Whenever
possible, the drug dosages that are analyzed are identical in both the human and
animal markets.(30)
This was the case with all of the drugs analyzed in this report except for
Lasix, Amoxil, and Bactroban. For these drugs, the closest dosage sizes
available in the two markets were chosen for comparison, and price comparisons
were based on costs per gram of active drug ingredient. The dosages used in this
study are shown in Appendices A and B.
Once a dosage size was selected, prices were
determined for the quantity of drug in a typical one month supply of the product
for human consumers. Information on typical quantities prescribed by physicians
was obtained from the Physicians Desk Reference or from the U.S.
Pharmacopeia Dispensing Information.
E. Evaluation
of Impacts on Uninsured Consumers
To determine the effect that manufacturer-level
price differentials could have on prices paid by uninsured consumers in the
5th Congressional District in Florida, the
minority staff and the staff of Rep. Thurman's congressional office conducted a
survey of twelve drug stores -- including both independent and chain stores --
in her district. Rep. Thurman represents the 5th Congressional District on the
western coast of Florida, which includes the cities of Gainesville, Inverness,
and New Port Richey.
III. RESULTS OF PRICE
COMPARISONS
A. Manufacturer
Prices Are Over Twice as High for Humans as for Animals
1. Price Differentials for Popular
Prescription Drugs
Eight brand name drugs among the top 200 human
drugs are approved for use through the same dosage route for both humans and
animals and are commonly obtained for human use via out-patient prescription.
For these eight popular drugs, drug manufacturers charge far more when the
intended end-users are humans than when the intended end-users are animals. The
average differential between the price at which the drugs are sold by
manufacturers for human use and the price at which the drugs are sold by
manufacturers for animal use is 106% to 151%. This means that drug manufacturers
charge more than twice as much for these drugs when sold for use by humans as
they charge when the drugs are sold for use by animals (Table 1).
| Table 1: Drug Manufacturers Charge More for
Popular Drugs Used by Humans than for the Same Drugs Used by
Animals. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Drug Name |
Manufacturer of Human
Version |
Human Use |
Manufacturer Price
(One Month Supply) |
Price
Differential |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Animal Market |
Human Market |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Bactroban |
SmithKline Beecham |
Antibiotic |
$9.98 |
$31.56 |
216% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Augmentin |
SmithKline Beecham |
Antibiotic |
$18.00 |
$56.40 |
213% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lodine |
American Home Products |
Arthritis |
$37.80 |
$108.90 |
188% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stadol |
Bristol Myers Squibb |
Pain Relief |
$25.48 |
$61.11 |
140% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lasix |
Hoechst Marion Roussel |
High Blood Pressure |
$4.80 |
$9.60 |
100% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Vasotec |
Merck |
High Blood Pressure |
$51.30 |
$78.55 |
53% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lanoxin |
Glaxo Wellcome |
Heart Failure |
$6.36 |
$25.65 ($4.08) |
303% (-56%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Amoxil |
SmithKline Beecham |
Antibiotic |
$16.20 |
$15.30 |
-6% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Average for Eight Drugs |
|
|
151% (106%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three drugs with high price differentials in
percentage terms are Bactroban, Augmentin, and Lodine (Figure 1). Bactroban and
Augmentin, which are manufactured by SmithKline Beecham for the human market,
are both antibiotics. Both had price differentials of over 200%, which means
that the manufacturer-level price for these drugs is over three times more
expensive when the drug is intended for human use than when the drug is intended
for animal use.
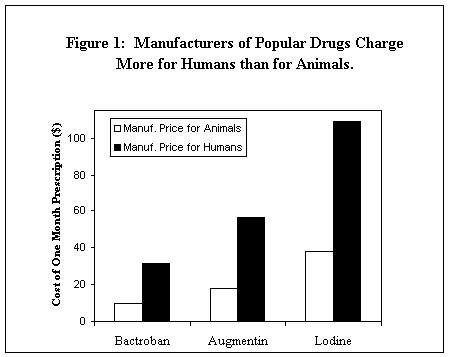
Lodine is an arthritis medication. A common
prescription for Lodine is ninety 300 mg. capsules. Fort Dodge, a subsidiary of
American Home Products, sells this quantity of Lodine for only $37.80 when the
intended end-users are dogs or cats. But when the intended end-users are humans,
Wyeth-Ayerst, another subsidiary of American Home Products, sells the same
quantity of the drug for $108.90 -- a price differential of 188%.
Two different price differentials are presented
in this report for Lanoxin, a medication used to treat heart failure in both
humans and dogs. For the animal market, Lanoxin is manufactured in a liquid
form. For the human market, it is manufactured in both a liquid form and a
tablet form. For this drug, the most direct price comparison is a
liquid-to-liquid comparison between the manufacturer-level price for the liquid
version of the drug for animals ($6.36) and the manufacturer-level price for the
liquid version of the drug for humans ($25.65). This "apples to apples"
comparison results in a price differential of 303%, the highest price
differential in percentage terms among the popular drugs analyzed in this
report.
It is also possible, however, to compare the
price of the liquid version of the drug for animals with the price of the tablet
version for humans by calculating costs per gram of active drug ingredient.
Although this approach can be criticized as an "apples to oranges" comparison,
it is also included in this analysis in an effort to be conservative. The
manufacturer-level price for the human version of the same quantity of the drug
in tablet form ($4.08) is less than the manufacturer-level price of either the
liquid version of the drug for animals or the liquid version of the drug for
humans, producing a human to animal price differential of -56%.(31)
The only popular drug for which the
manufacturer-level price is less expensive for humans than for animals in a
direct "apples to apples" comparison is Amoxil. For this drug, which is
manufactured by SmithKline Beecham for the human market, the manufacturer-level
price is 6% lower for the human market than for the animal market.
- Price Differentials for Directly
Comparable Prescription Drugs
This report found similar results when analyzing the
pricing of directly comparable drugs: manufacturers charge significantly more
for directly comparable brand name drugs when the drugs are used by humans than
when the drugs are used by animals. There are eight brand name drugs that are
approved for use in the same dosage in both humans and animals, are manufactured
for both markets by the same (or related) companies, and are commonly obtained
for human use via out-patient prescription. For these eight products, the
average differential between the price at which the drug is sold by the
manufacturer for human use and the price at which the drug is sold by the
manufacturer for animal use is 131% (Table 2). This price differential is
similar to the average price differential observed for the eight popular drugs.
For both sets of drugs, manufacturers charge an average of more than twice as
much when a drug is sold for use by humans than they charge when the same drug
is sold for use by animals.
Among the directly comparable drugs, Medrol,
which is manufactured by Pharmacia and Upjohn, has the highest price
differential: 415%. This drug is used to treat arthritis, asthma, and allergies
in humans and is used as an anti-inflammatory agent in dogs and cats. Pharmacia
and Upjohn charges $20.10 for a one month supply of Medrol when the end-user is
a person seeking treatment for arthritis, but only $3.90 for the same quantity
of Medrol when the end-user is a dog. Winstrol, which is manufactured by Sanofi,
has the second highest price differential: 256%. This drug is used to treat
end-stage renal disease and anemia in humans and weight loss, debility, and
other symptoms associated with old age in dogs and cats. Sanofi sells this drug
for $19.20 when the end-users are humans, but only $5.40 when the end-users are
animals.
| Table 2: Drug Manufacturers Charge More for Directly
Comparable Drugs When the Drugs Are Used by Humans than When the Drugs Are
Used by Animals. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Drug Name |
Manufacturer |
Human Use |
Manufacturer Price
(Monthly Supply) |
Price Differential |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Animal Market |
Human Market |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Medrol |
Pharmacia and Upjohn |
Arthritis; Allergies; Asthma |
$3.90 |
$20.10 |
415% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Winstrol |
Sanofi |
Anemia; Renal Disease |
$5.40 |
$19.20 |
256% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lodine |
American Home Products |
Arthritis |
$37.80 |
$108.90 |
188% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Robaxin |
A.H. Robins |
Pain Relief |
$15.00 |
$31.20 |
108% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Vasotec |
Merck/Merial |
High Blood Pressure |
$51.30 |
$78.55 |
53% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cleocin |
Pharmacia and Upjohn |
Antibiotic |
$17.10 |
$22.20 |
30% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Robinul |
A.H. Robins |
Ulcers |
$29.40 |
$29.98 |
2% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fulvicin U/F |
Schering |
Antifungal |
$38.40 |
$36.60 |
-5% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Average for Eight Drugs |
|
|
131% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Overall, manufacturers charge more for seven of the eight
directly comparable drugs when the end users are humans than they charge when
the end users are animals. The only drug that was less expensive at the
manufacturer-level for humans than for animals was Fulvicin U/F, which is
manufactured by Schering.
B.
Price Differentials Can Be Substantial in Dollar Terms
These price differences can translate into large
differences in dollar terms. Two of the drugs surveyed in this report, Lodine
and Vasotec, are both popular (among the top 200 human drugs in 1998) and
directly comparable (manufactured in the same dosage route and dosage by the
same or related companies in both the human and animal markets). Both have large
price differentials in dollar terms.
Lodine, the arthritis medication made by
subsidiaries of American Home Products, has the largest price differential in
dollar terms observed in this report. The manufacturer sells a monthly
prescription of this drug for $108.90 for human use -- more than $70 more than
the manufacturer charges when selling the same quantity of the drug for use by
dogs or cats.
Similarly, Vasotec, the 14th most prescribed
human drug in 1998, has a large dollar value price differential. Merck sells a
one-month supply of Vasotec for $78.55 when the intended users are human, while
Merial, a Merck subsidiary, charges only $51.30 - over $25 less - when the
intended users are animals. Vasotec treats a chronic condition (high blood
pressure) and is often taken over long periods by senior citizens and others. On
an annual basis, the manufacturer-level price differential for Vasotec is over
$325.
C.
Retail-Level Price Differentials Between Human and Animal DrugsCan Be
Significant
This analysis focuses primarily on the prices
that drug manufacturers charge wholesalers of human and animal drugs, not the
prices that wholesalers charge pharmacists and veterinarians or the prices that
pharmacists and veterinarians charge individual consumers. In the case of
comparisons of human and animal drugs, a manufacturer-level price comparison
provides a more direct comparison than a retail-level price comparison because
of the differing nature of the retail market for human and animal drugs. Human
drugs are prescribed by a doctor and dispensed by a pharmacist. In contrast,
most animal drugs are both prescribed and dispensed by the veterinarian. Because
they serve both functions, veterinarians often incorporate some of the costs of
practicing veterinary medicine into their prices for prescription drugs,
resulting in markups that are many times higher than pharmacists charge.
Because of these complexities, the most direct
comparison of retail-level prices can be obtained by comparing the prices that
pharmacists charge human consumers with the prices that specialized veterinary
pharmacies charge animal owners. Through the Internet and mail order, the
minority staff identified three veterinary pharmacies that resemble human
pharmacies in that they dispense, but do not prescribe, medications.(32)
These veterinary pharmacies sell seven of the eight popular drugs investigated
in this report. The report compared the average prices that these veterinary
pharmacies charge for the drugs with the average retail prices that consumers in
Rep. Thurman's district must pay for the same drugs.
This analysis showed that there is a substantial
difference between what consumers in Florida must pay for these seven drugs and
the prices that animal owners can pay to acquire the drugs from veterinary
pharmacies.(33)
On average, it costs consumers in the 5th Congressional District in
Florida107% to 198% more for these drugs than animal owners (Table 3). This
means that uninsured consumers in Rep. Thurman's district, such as senior
citizens without prescription drug coverage, are being forced to pay on average
two to three times as much as animal owners to acquire these drugs.
| Table 3: Retail-Level Prices Are More Expensive for Human
Consumers in Rep. Thurman's District than for Animal Owners. |
|
|
|
|
| Drug |
Average Retail
Price
(Monthly Supply) |
Price Differential
(%) |
|
Price at Veterinary
Pharmacy |
Retail Price for
Humans |
|
|
|
|
|
| Bactroban |
$14.50 |
$47.80 |
230% |
| Augmentin |
$25.84 |
$80.53 |
212% |
| Lasix |
$5.37 |
$15.72 |
193% |
| Lodine |
$65.25 |
$136.72 |
110% |
| Amoxil |
$15.29 |
$19.97 |
31% |
| Lanoxin |
$5.41 |
$38.43 ($4.39) |
610% (-23%) |
| Vasotec |
$91.41 |
$91.85 |
0% |
| Average Price Differential |
|
198% (107%) |
V. DRUG PRICES FOR UNINSURED
CONSUMERS IN THE 5TH CONGRESSIONAL DISTRICT IN FLORIDA COULD BE SIGNIFICANTLY
REDUCED BY PREVENTING PRICE DISCRIMINATION
The price comparisons described in part III show
that drug manufacturers charge different prices for drugs intended for human use
than for drugs intended for animal use. This part assesses the impact that this
manufacturer-level price discrimination has on human drug prices for consumers
in Rep. Thurman's district.
To make this assessment, the report estimates
the potential cost savings for consumers in the 5th Congressional District in
Florida if drug manufacturers did not engage in price discrimination and instead
charged the same price for human drugs that they now charge for animal drugs.
Experts state that drug wholesalers and retail pharmacies are highly competitive
and are likely to pass any cost savings on to consumers. According to Professor
Stephen W. Schondelmeyer, "[a]ny discounts passed on to community pharmacies
will be passed on to the consumer, or payor, of the prescription because of the
competitive retail environment."(34)
For this reason, the analysis assumes that the reductions in the
manufacturer-level price will be passed on to the pharmacist by the human drug
wholesaler and to the consumer by the pharmacist.
Under these circumstances, the potential savings
would be substantial for consumers in Rep. Thurman's district who purchase their
own drugs, such as senior citizens without prescription drug insurance. For
example, the average retail cost of purchasing a one month supply of the blood
pressure medication Vasotec in the 5th district is $91.85.
If this price were reduced by $27.25, which is the difference between the
manufacturer-level price for human use and the manufacturer-level price for
animal use, a consumer in the 5th district would pay only $64.60 for a one-month
supply. On an annual basis, the consumer in Rep. Thurman's district would save
over $325.
In dollar terms, consumers in Rep. Thurman's
district who purchase Lodine would realize the greatest savings. Their drug
costs would drop by over $70 for a one-month supply. Table 4 summarizes the
potential savings for each of the eight popular drugs analyzed in this report.
The average savings for the eight popular drugs would be 24% to 37%.
| Table 4: Consumers in Rep. Thurman's District Could Save
Hundreds of Dollars if Drug Manufacturers Did Not Engage in Price
Discrimination. |
|
|
|
|
| Drug |
Average
Retail Price
in the 5th
District
(Monthly
Supply) |
Potential
Savings
(Dollars) |
Potential
Savings
(%) |
|
|
|
|
| Lodine |
$136.72 |
$71.10 |
52% |
| Augmentin |
$80.53 |
$38.40 |
48% |
| Bactroban |
$47.80 |
$21.58 |
45% |
| Stadol |
$84.51 |
$35.63 |
42% |
| Lasix |
$15.72 |
$4.80 |
31% |
| Vasotec |
$91.85 |
$27.25 |
30% |
| Lanoxin |
$38.43
($4.39) |
$19.29
(-$2.28) |
50% (-52%) |
| Amoxil |
$19.97 |
-$0.90 |
-5% |
| Average
Savings |
37% (24%) |
These estimates of the potential savings for
uninsured consumers in Rep. Thurman's district should be considered upper-bound
estimates. The estimates assume that drug manufacturers sell their products to
human drug wholesalers at the same price that they are now selling their
products to animal drug wholesalers. In reality, drug manufacturers could choose
to sell to both markets at a price between their current animal and human
prices. Moreover, representatives of the drug manufacturers have argued that
pharmacists would not pass along all of the cost savings to uninsured consumers.
These factors could reduce the potential savings.
VI. QUALITY DIFFERENCES AND
RESEARCH COSTS DO NOT APPEAR TO EXPLAIN THE PRICE DIFFERENTIALS
This report examined several possible
explanations for the substantial manufacturer-level price differences observed
between the drugs intended for human use and the drugs intended for animal use.
The most probable explanation appears to be that price discrimination is a
central component of the drug manufacturers' pricing strategies.
A. Drug
Quality and Production Costs
It appears unlikely that differences in drug
quality can explain the results observed in this study. The Food and Drug
Administration regulations governing drug quality and production, the so-called
"good manufacturing practice" (GMP) requirements, are codified in 21 C.F.R. part
211. These requirements, which are designed to ensure drug quality and
consistency, apply equally to both human and animal drugs. According to
FDA:
The methods, facilities, and controls under
which animal drugs are manufactured, processed, packaged, or held for sale must
conform to the requirements of the regulations for Current Good Manufacturing
Practices in the drug industry generally.(35)
Differences in production costs are also
unlikely to be the cause of the high price differentials because production
costs are only a small part of the final cost of a prescription drug. The
typical marginal cost of manufacturing additional volumes of a medication has
been estimated to be only 5% of the retail cost.(36)
Thus, even large differences in drug production costs would be unlikely to
result in the differences in drug prices observed in this study.
B. Research and
Development Costs
Drug manufacturers frequently point to the costs
of research and development as a justification for high prices. However,
differences in research and development costs do not appear to explain the
differences in cost between identical human and animal drugs.
According to an industry expert consulted by the
minority staff, Dr. Alan Sager of the Boston University School of Public
Health:
The observed price differences cannot be
explained by differences in research costs. Research is a fixed or sunk cost.
Manufacturers do not set their prices based on recovery of these costs. Instead,
they set their prices as high as possible in order to maximize revenue and
profit.(37)
Moreover, according to industry analysts,
pharmaceutical manufacturers are "investing heavily" in research and development
of animal drugs.(38)
Relative to the size of the markets, drug manufacturers appear to spend
approximately as much on research and development of animal drugs as they do on
research and development of human drugs. Pfizer, an industry leader in animal
drug sales, had revenues of $1.3 billion from animal drugs in 1998, and spent
approximately $200 million - over 15% of total sales - on research of animal
drugs.(39)
Pfizer also spent approximately the same proportion of revenues, 17%, on its
drug products intended for sale to humans.(40)
The high research investment in animal drugs is
confirmed by the pharmaceutical industry, which states:
All animal health products go through a
stringent seven-step process that involves testing to discover a product,
testing to approve the product, and testing to monitor the product once it's
been approved....Bringing an animal health product to market is a complex
process. Only one in 20,000 discovered chemicals ever makes it from the
laboratory to the farm. And only one in 200 potential drugs makes it through
pre-clinical testing and approval.(41)
VI.
CONCLUSION
The findings in this report are consistent with
the results of the first drug pricing study done at Rep. Thurman's request. The
first study found that uninsured seniors in the 5th Congressional District in
Florida pay 112% more for the five most popular prescription drugs used by
seniors than favored purchasers such as HMOs and the federal government.(42)
When this study was updated in March 2000, the price differential between
seniors and favored purchasers increased to 125%.(43)
The second study found that uninsured seniors in the 5th Congressional District
in Florida pay 81% more for these prescription drugs than individual purchasers
in Canada, and 79% more than individual purchasers in Mexico.(44)
All three studies thus reach the same basic finding: drug manufacturers are
engaged in systematic price discrimination that adversely affects millions of
senior citizens and other consumers who lack prescription drug coverage.
Appendix A:
Information on Popular Prescription Drugs in this Survey
| Human Brand Name |
Animal Brand Name |
Tablet, Capsule, or Package
Size |
Average Manufacturer Charge
per Tablet, Capsule, or Package |
Average Manufacturer Charge
per Gram of Active Ingredient |
Typical One Month Prescription for
Humans |
Average Manuf. Charge for
Quantity of Active Ingredient in Typical One Month Human
Prescription |
|
|
|
Animal |
Human |
Animal |
Human |
|
Animal |
Human |
| Amoxil |
Robamox |
250 mg. cap. (human); 200 mg. cap. (animal) |
$0.14/cap. |
$0.17/cap. |
$0.70 |
$0.68 |
90 cap. |
$16.20 |
$15.30 |
| Augmentin |
Clavamox |
125 mg. tab. |
$0.30/tab. |
$0.94/tab. |
$2.40 |
$7.52 |
60 tab. |
$18.00 |
$56.40 |
| Bactroban |
Bactoderm |
2%, 30 gm. (human); 2%, 15 gm. (animal) |
$4.99/
15 gm. |
$31.56/
30 gm. |
$16.63 |
$52.60 |
30 gm. |
$9.98 |
$31.56 |
| Lanoxin (liquid)
Lanoxin (tab.) |
Cardoxin LS
NA |
50 g./ml., 2 oz.
0.125 mg. |
$6.36
NA |
$25.65
$0.17/tab. |
$2.12
NA |
$8.55
$1.36 |
3 gm. active ingredient
30 tab. |
$6.36
N/A |
$25.65
$5.10(45) |
| Lasix |
Lasix |
20 mg. tab. (human); 12.5 mg. tab. (animal) |
$0.05/cap. |
$0.16/cap. |
$8.00 |
$4.00 |
60 cap. |
$4.80 |
$9.60 |
| Lodine |
Etogesic |
300 mg. cap./tab. |
$0.42/tab. |
$1.21/cap. |
$1.40 |
$4.03 |
90 cap. |
$37.80 |
$108.90 |
| Stadol |
Torbugesic |
2 mg./ml., 10 ml. |
$25.48 |
$61.11 |
$1274.00 |
$3055.50 |
10 ml. |
$25.48 |
$61.11 |
| Vasotec |
Enacard |
5 mg. tab. |
$0.57/tab. |
$0.88/tab. |
$114.00 |
$176.00 |
90 tab. |
$51.30 |
$78.55 |
Appendix B: Information on
Directly Comparable Prescription Drugs in this Survey
| Human Brand Name |
Animal Brand Name |
Tablet or Capsule
Size |
Average Manufacturer Charge
per Capsule or Tablet |
Average Manufacturer Charge
Per Gram of Active Ingredient |
Typical One Month
Prescription
For Humans |
Average Manuf. Charge for
Quantity of Active Ingredient in Typical One Month Human
Prescription |
|
|
|
Animal |
Human |
Animal |
Human |
|
Animal |
Human |
| Cleocin |
Antirobe |
75 mg. |
$0.57 |
$0.74 |
$7.60 |
$9.87 |
30 cap. |
$17.10 |
$22.20 |
| Fulvicin U/F |
Fulvicin U/F |
500 mg. |
$1.28 |
$1.22 |
$2.56 |
$2.44 |
30 cap. |
$38.40 |
$36.60 |
| Lodine |
Etogesic |
300 mg. |
$0.42 |
$1.20 |
$1.40 |
$4.00 |
90 cap. |
$37.80 |
$108.90 |
| Medrol |
Medrol |
4 mg. |
$0.13 |
$0.67 |
$32.50 |
$167.50 |
30 tab. |
$3.90 |
$20.10 |
| Robaxin |
Robaxin |
500 mg. |
$0.25 |
$0.51 |
$0.50 |
$1.02 |
60 tab. |
$15.00 |
$31.20 |
| Robinul |
Robinul-V |
1 mg. |
$0.49 |
$0.50 |
$490.00 |
$500.00 |
60 tab. |
$29.40 |
$29.98 |
| Vasotec |
Enacard |
5 mg. |
$0.57 |
$0.87 |
$114.00 |
$174.00 |
90 tab. |
$51.30 |
$78.55 |
| Winstrol |
Winstrol-V |
2 mg. |
$0.18 |
$0.64 |
$90.00 |
$320.00 |
30 tab. |
$5.40 |
$19.20 |
1. Health Care Finance
Administration, National Health Expenditures (1999) (online at
www.hcfa.gov/stats/nhe-oact/tables/t10.htm).
2. National Institute for
Health Care Management Foundation, Factors Affecting the Growth of
Prescription Drug Expenditures (July 9, 1999).
3. National Health
Expenditures, supra note 1.
4. Senate Special Committee On
Aging, Developments in Aging: 1993, 103d Cong., 2d Sess. 35 (1994) (S.
Rpt. 403).
5. 5 National
Economic Council, Domestic Policy Council, Disturbing Truths and Dangerous
Trends: The Facts About Medicare Beneficiaries and Prescription Drug
Coverage (July 22, 1999). In this study, private sector retiree coverage was
considered to be the only dependable form of private-sector prescription drug
coverage for senior citizens. Other sources of coverage, such as Medigap
coverage or Medicare managed care plans were not considered dependable because
the plans are often expensive, inaccessible, or inadequate.
6. Health Affairs, Medicare
Beneficiaries and Drug Coverage, 252 (Mar./Apr. 2000).
7. AARP Public Policy
Institute, Out-of-Pocket Health Spending by Medicare Beneficiaries Age 65 and
Older: 1999 Projections (Dec. 1999).
8. See National Economic
Council, Domestic Policy Council, Disturbing Truths and Dangerous Trends: The
Facts About Medicare Beneficiaries and Prescription Drug Coverage,
supra note 5; Soumerai, Steven, Inadequate Prescription-Drug Coverage
for Medicare Enrollees - A Call to Action, New England Journal of Medicine
(Mar. 1999).
9. Families USA Foundation,
Worthless Promises: Drug Companies Keep Boosting Prices 6 (March
1995).
10. See Health Affairs,
Drug Coverage and Drug Purchases by Medicare Beneficiaries With
Hypertension, 219-230 (Mar./Apr. 2000).
11. Congressional Budget
Office, How Increased Competition from Generic Drugs Has Affected Prices and
Returns in the Pharmaceutical Industry, xi (July 1998).
12. Federal Trade Commission,
The Pharmaceutical Industry: A Discussion of Competitive and Antitrust Issues
in an Environment of Change, 75 (Mar. 1999).
13. Minority Staff Report of
the House Committee on Government Reform, Prescription Drug Pricing in the
5th Congressional District in
Florida: Drug Companies Profit at the
Expense of Older Americans (May 1999);
Minority Staff Report of the House Committee on Government Reform,
Prescription Drug Pricing in the 5th Congressional District in Florida: Drug Companies Profit at the Expense of Older
Americans (updated Mar. 2000).
14. Minority Staff Report of
the House Committee on Government Reform, Prescription Drug Pricing in the
5th Congressional District in
Florida: An International Price Comparison
(Aug. 1999).
15. FDA, Approved Drug
Products with Therapeutic Equivalence Evaluations (1999).
16. Id.
17. Factors Affecting the
Growth of Prescription Drug Expenditures, supra note 2.
18. FDA, FDA Approved
Animal Drug Products (1999).
19. Id.
20. See Tanouye, Elyse, Wall
Street Journal, The Ow in Bowwow: With Growing Market in Pet Drugs, Makers
Revamp Clinical Trials (Apr. 13, 1999).
21. Animal Health Institute,
Press Release: Animal Health Product Sales Rise to $4.3 Billion in 1998
(July 31, 1998).
22. For example, Prozac is
prescribed by some veterinarians to treat anxiety disorders in dogs. See The
Ow in Bowwow: With Growing Market in Pet Drugs, Makers Revamp Clinical
Trials, supra note 20. FDA regulations allow veterinarians to
prescribe a human-approved drug for animal use, provided that a product
containing the same active ingredient has not also been approved for use in
animals.
23. This report focused on
brand name drugs because manufacturers of brand name drugs generally have
greater control over drug pricing than manufacturers of less expensive generic
drugs. Several of the drugs included in the survey, however, are also available
in generic versions. Consumers who purchase these drugs in their generic version
are likely to pay less than those who purchase the brand name version. The
Congressional Budget Office has found that the availability of a generic drug
often does not decrease the cost of the brand name product. See How Increased
Competition from Generic Drugs Has Affected Prices and Returns in the
Pharmaceutical Industry, supra note 11.
24. Sometimes, the same drug
may be taken by humans and animals through different dosage routes. For example,
the drug may be taken orally by humans (through a tablet or capsule) and by
injection by animals. Only drugs that are taken through the same dosage route
are included in this analysis to increase the comparability of the drugs.
25. Pharmacy Times, The Top
200 Drugs of 1998 (1999) (online at
http://www.pharmacytimes.com/top200.html).
26. Lasix is sold in 20 mg.
tablets for the human market and in 12.5 mg. tablets for the animal market.
Amoxil is sold in 250 mg. capsules for the human market and 200 mg. capsules for
the animal market. Bactroban is sold in 30 gram tubes for the human market and
15 gram tubes for the animal market.
27. Although Winstrol is
manufactured by Sanofi, it is sold by Pharmacia and Upjohn on the animal market.
Pharmacia and Upjohn, Animal Health Products: Winstrol-V (1999) (online
at www.pnuanimalhealth.com/product/companimal/winstf2.html).
28. Patricia M. Danzon,
Price Comparisons for Pharmaceuticals: A Review of U.S. and Cross-National
Studies (April 1999).
29. The report adjusted for
the wholesale markup by reducing the average wholesale-level price by 3%. This
represents the midpoint of the markup that human wholesalers typically charge
pharmacies. See Price Comparisons for Pharmaceuticals: A Review of U.S. and
Cross-National Studies, supra note 28. Because the veterinary market
is smaller than the human market, it is possible that wholesalers of animal
drugs charge a higher markup than wholesalers of human drugs. If animal drug
wholesalers charge higher markups than human drug wholesalers, the actual
manufacturer-level prices for animal uses would be lower than the level reported
in this study. This would make the actual level of manufacturer price
discrimination higher than reported in this study.
30. In some cases, the drugs
analyzed in the report are sold in a range of different dosages on the human
market. For example, Lodine is sold for humans in dosages of 200, 300, 400, and
500 mg. In these cases, prices were compared based on the closest dosages
available in both the human and animal markets. In the case of Lodine, the drug
is only available in the 300 mg. dosage for animals. Thus, for purposes of the
price comparisons in this report, the price comparison is based on the 300 mg.
human version of Lodine.
31. A similar cost-per-gram
analysis comparing the price of the tablet form of a drug to the price of the
liquid form could also be done in the case of Lasix, which is sold in tablet
form for animals and in both tablet form and liquid form for humans. In the case
of Lasix, including this "apples to oranges" comparison would have the opposite
effect: it would substantially increase the price differential between the
animal and human versions of the drug. This analysis was not included in the
report.
32. The three veterinary pharmacists were KV Vet Supply,
Vet Warehouse, and Lambriar Animal Health.
33. Average retail prices for the tablet human version of
Lanoxin were not collected in Rep. Thurman's
district. As a substitute, the report uses the price at which the tablet version
of Lanoxin is available through a major Internet pharmacy. Generally, prices in
Rep. Thurman district are slightly higher
than prices available at this Internet pharmacy.
34. Schondelmeyer, Stephen W.,
PRIME Institute, University of Minnesota, Competition and Pricing
Issues in the Pharmaceutical Market, University of Minnesota, 12 (Aug.
1994).
35. U.S. Food and Drug
Administration, Requirements of Laws and Regulations Enforced by the U.S.
Food and Drug Administration (1999) (online at http://www.fda.gov/opacom/
morechoices/smallbusiness/blubook.htm#animalprod.html).
36. Alan Sager and Deborah
Socolar, Affordable Medications for Americans (July 27, 1999).
37. Dr. Alan Sager, Boston
University School of Public Health, Response to Rep. Henry A. Waxman
(Aug. 1999).
38. Newsweek, When Pets Pop
Pills (Oct. 11, 1999).
39. Pfizer, Inc., 1998
Annual Report (1999); Los Angeles Times, Animal Drugs Become Big Pet
Project for Industry (Oct. 12, 1999).
40. Pfizer, Inc., 1998
Annual Report (1999).
41. Animal Health Institute,
Testing...testing....testing: Food Safety and Animal Drugs (May
1999).
42. Prescription Drug
Pricing in the 5th Congressional District in Florida: Drug Companies Profit at
the Expense of Older Americans (May 1999), supra note 12.
43. Prescription Drug
Pricing in the 5th Congressional District in Florida: Drug Companies Profit at
the Expense of Older Americans (Mar. 2000), supra note 12.
44. Prescription Drug
Pricing in the 5th Congressional District in Florida: An International Price
Comparison, supra note 13.
45. The manufacturer-level
price for thirty 0.125 mg Lanoxin tablets is $5.10. These 30 tablets contain a
total of 3.75 grams of active ingredient. The liquid version of the product
contains 3 grams of active ingredient. In order to obtain a direct comparison of
prices for these products, the analysis determined the cost of 3 grams of active
ingredient in the tablet form. This cost, which is the price used in Table 1 in
the text, is (3/3.75) *($5.10) = $4.08.
Return to
the Prescription Drug Study

