Medicare Coverage Policy ~ DecisionsUltrasound Stimulation for Nonunion Fracture Healing (#CAG-00022)Decision Memorandum
This memo serves four purposes: (1) outlines the physiology of normal fracture healing; (2) reviews the history of Medicare’s coverage policy on ultrasound stimulation for fracture healing; (3) analyzes the relevant scientific data related to the use of ultrasound osteogenic bone stimulators for the treatment of nonunion; (4) delineates the reasons for making a positive national coverage decision. Normal fracture healing Fractures are common injuries with over 6.8 million occurring yearly in the United States. Over 900,000 fractures each year result in hospitalization with over half of these hospitalized patients over 65 years old. Women over 65 years are three times as likely to suffer a fracture than their male counterparts, with the majority of these injuries affecting the hip or distal forearm. The body is normally able to effectively heal fractures with conservative medical treatment. Like most biological processes, normal fracture healing is complex and involves the integrated action of numerous cells, genes, and minerals. The process can be simplified into three main stages – inflammatory, reparative, and remodeling.
Reparative: A soft callus of fibroblasts (containing collagen), osteoblasts, and chondroblasts (which build cartilage) begins to form within two weeks after injury. Type II collagen is the main component of the cartilage matrix that initially begins to bridge the fracture gap. Via a process called endochondral ossification, the soft callus transforms into a hard callus of woven bone. Woven bone (immature bone with random collagen and bone cell orientation) can develop into a bony union within 2-3 months postfracture.Remodeling: Through the action of osteoclasts and osteoblasts, the initial woven bone is structurally modified to lamellar bone. Lamellar bone consists of highly organized mineral plates designed to withstand maximum stress. This final phase could take years, resulting in a bone that may be thicker and stronger than the original.In addition to the local cell recruitment and gene expression, many other factors are involved in the healing of a fracture. The severity of the fracture, the nature of the blood supply, the extent of soft tissue damage, the involvement of a tumor, or infection can alter the successful union of a fracture. Health considerations such as diabetes, vascular insufficiency, osteoporosis, anemia, hormone deficiency, smoking, age, or certain medications can also affect the quality and rate of fracture healing. Because of the comorbidities associated with aging, health considerations are especially important for the Medicare population who sustain fractures. Definition of nonunion Even with standard orthopedic management, 5-10% of fractures do not heal as well or as quickly as expected. These healing disorders are designated as delayed union or nonunion.
Nonunion of a long bone fracture must be documented by a minimum of two sets of radiographs obtained prior to starting treatment with the osteogenesis stimulator, separated by a minimum of 90 days, each including multiple views of the fracture site, and with a written interpretation by a physician stating that there has been no clinically significant evidence of fracture healing between the two sets of radiographs.1 Current treatment of nonunion Treatment options for nonunion range from various surgical techniques to noninvasive electrical, electromagnetic, or ultrasound stimulation. Surgical techniques may include Ilizarov external fixation, intermedullary nails, compression plates, screws, pins, or bone grafts. Reported success rates for surgical treatment of nonunion have ranged from 68-96%. Overall, successful treatment of a nonunion often depends on reduction of the fracture, bone grafting if necessary, and stabilization (internal or external fixation). Electrical, electromagnetic, or ultrasound stimulation may be options for cases in which surgery is not an option. These methods may require the implantation of electrodes or external application of the device. It has been shown that mechanical or electrical stimuli can send regulatory signals to the bone causing physical remodeling of the tissue. Ultrasound treatment is used in conjunction with stabilization of the fracture, and the therapy is used both as an alternative to surgery or as a second therapeutic option after surgical failure. Specific biological effects have been attributed to ultrasound stimulation including increased signaling pathways in osteoblasts, increased release of growth factors, increased blood flow to the fracture site, and an increased expression of the aggrecan gene. Aggrecan is a proteoglycan that incorporates with the Type II collagen during the reparative phase of fracture healing. History of Medicare’s Coverage Policy on Ultrasound for Fracture Healing Ultrasound osteogenic stimulators fall under the benefit category of durable medical equipment (DME). Currently, the Medicare program does not cover ultrasound therapy for fracture healing. Coverage Issues Manual section 35-48 states:
The ultrasound device consists of two main components – a signal generator and a small, square transducer connected to the generator. Though the definite biological effects of the ultrasound are not clear, the device does not substantially increase the temperature of the tissue and operates at an intensity of 30 mW/cm2, nearly one hundred times less than some diagnostic ultrasound. Its effects are generally attributed to the physical signal delivered to the bone by the acoustic waves, though other biological mechanisms have been proposed. Some evidence, though limited, is available to support the effects of ultrasound on all three phases of fracture healing. Patients typically apply the device to the fracture site 20 minutes/day until the fracture is healed or the prescribing physician stops the treatment. Currently, Exogen, Inc.2 is the only manufacturer of an ultrasound osteogenic bone stimulator. They initially received Food and Drug Administration (FDA) approval (Pre- market approval) in October 1994 for the use of the ultrasound stimulator for the treatment of fresh fractures (less than 7 days). In August 1996, the Technology Advisory Committee (TAC), of the Health Care Financing Administration (HCFA), reviewed available data relating to ultrasound treatment and found insufficient evidence for effectiveness in the Medicare population. The TAC was also concerned that the available studies excluded patients who were receiving anticoagulants, prescription non-steroidal anti-inflammatory medications (NSAIDs) or calcium channel blockers, those with a history of thrombophlebitis, vascular insufficiency, alcoholism or nutritional deficiency. At that point, HCFA revised the Coverage Issues Manual section 35-48 to issue a noncoverage policy of ultrasound for all indications. In November 1998, ultrasound for the treatment of fresh fractures was revisited. The national noncoverage decision was officially reviewed and re-issued. HCFA determined that there was a lack of functional benefits attributable to ultrasound therapy, a failure to determine effectiveness in the elderly population, and a failure to demonstrate the prevention of delayed union or nonunion. Of note, this decision memorandum only addresses the topic of ultrasound stimulation for the treatment of established nonunions. The noncoverage policy for fresh fractures is not addressed in this document. Recent Developments and Timeline of Activities
Analysis of Scientific Data For a detailed review of the materials examined, refer to Appendix A. The materials include peer-reviewed articles, unpublished articles, and conference proceedings. Exogen provided HCFA with three published articles, one published review and a summary of the safety and effectiveness data presented to the FDA (which included 2 unpublished articles) for the ultrasound treatment of nonunions. Additionally, a Medline search with the terms "ultrasound," "fracture," "fracture healing," "nonunion," and "osteogenesis" was performed and returned an additional case report on the use of ultrasound for nonunion. HCFA also obtained one unpublished review, and, in addition, six unpublished articles that were analyzed for this decision. Issues Related to Coverage of Ultrasound Stimulation for Nonunion In addressing the national noncoverage policy regarding the use of ultrasound osteogenic stimulators for the treatment of nonunion fractures, the following questions arise:
In order to address the adequacy of the evidence, several issues need to be discussed. The first issue is study design. It is important to recognize that there is a hierarchy of evidence based on the methodology employed in a study. The most rigorous, well-designed studies are prospective, randomized clinical trials; however, such studies are not possible fin every clinical situation. The studies for the ultrasound treatment of nonunions are all case series, with some statistical analysis using self-paired controls within those populations. Investigators have asserted that receiving approval from an Institutional Review Board (IRB) for a nonunion study involving placebo devices would be difficult. HCFA acknowledges the concerns associated with providing placebo to nonunion fractures that, by their own definition, have no sign of healing without some active intervention. Therefore, although preferable, it might be impractical to require manufacturers to conduct placebo-controlled studies on this patient population. There are, however, better, alternative study designs which should have been considered. For example, the ultrasound stimulator could be compared to the other noninvasive treatments or to surgical intervention. Such a comparative analysis would be of interest to HCFA. One of the potential problems with the study designs used for the evaluation of ultrasound treatment for nonunions stems from the fact that all the studies are retrospective. Patients were entered into the studies based on certain inclusion/exclusion criteria, but those criteria were not developed a priori; rather, they were initiated after the patients had already been treated. The fact that the patients for the study were selected only from patients that had been treated by the device precludes these studies from being considered historically prospective. By designing a study after the patients and their outcomes are documented, the study design could introduce a systematic bias. Which types of patients were initially prescribed the device? Were these patients representative of the patient population that would ultimately use the device? Was there anything distinct about the fractures treated with the device? An added concern is that all reports involving US patients are based on Exogen registry data accumulated since October 1994.3, 4 Registry data has the potential for bias because the registry could contain only selected patients that meet certain criteria. Because of such bias, it is often difficult for registries to demonstrate causality. The sufficiency and adequacy of registry data needs to be determined on an individual basis. In this series of studies, bias is reduced since all patients treated with the device in the United States since October 1994 have been documented in the registry regardless of fracture type, compliance, or outcome.5 For the decision relating to fresh fractures, the registry was not sufficient evidence on which to base a coverage decision. The registry data did not provide comprehensive information about functional and clinical outcomes in the fresh fracture population (cast removal, return to work time, time to weightbearing), nor did it provide appropriate statistical analyses of variables; therefore, it did not represent adequate evidence for that population. For the nonunion population, however, the outcome of healed/failure to heal is a clinically relevant outcome that is provided by the registry. A further problem with the registry data is the overlap between studies. The studies that analyze the registry data often look at populations including the same patients. For instance, the Heppenstall study contains patients entered in the registry between October 17, 1994 and October 17, 1996, and a Mayr article studies patients entered in the registry between October 17, 1994 and July 14, 1997. The strength of the overall body of evidence is weakened by the fact that the individual studies use many of the same patients. In an effort to validate the results from the registry, Mayr. et al., in two separate studies of the registry population conducted at different times, compared data from a separate German population to the United States data.6 The German population was composed of consecutive patients treated with the ultrasound device in clinics around Germany and was part of a study later published by Gebauer et al. The German study followed additional inclusion criteria to limit confounding factors that may contribute to healing. The cases were all reviewed by the investigators to ensure that healing had stopped and that the last surgical intervention was at least two months before ultrasound treatment. Comparisons were then conducted based on nonunion heal rate, average healing time, average fracture age, and median fracture age. A student’s t-test comparing average healing time and average fracture age between groups showed no significant difference; the nonunion healed rates were similar (83% US v. 93% German and 86% US v. 94% German), but statistical analysis was not provided. Another concern related to study design relates to the statistical analysis. The studies reviewed for this decision either simply report a percent of patients healed or present a statistical comparison using the patient as his/her own control. For the statistical comparisons, the authors assume a healed rate of £ 5% as the null hypothesis.7 For the studies that computed a statistical comparison, the p-values were significant (p£ 0.00001) both for overall results and intention-to-treat groups. The self-paired analysis is appropriate in this case and limits potential significant differences between treatment and control groups. Given the concerns about placebo devices and the limited alternatives for studying this population, the use of registry data, as well as self-paired, retrospective study design is minimally acceptable for determining the effectiveness of the ultrasound device for the treatment of nonunion. The second issue when assessing the adequacy of evidence is the consistency of the results. If the studies available on a certain technology are inconsistent, the actual effectiveness of the device is difficult to determine. The articles reviewed for this decision are fairly consistent both in terms of design and outcome. All the studies use an outcome of healed versus failure to heal; they all require a fracture age of at least six months; and they all utilize ultrasound treatment without concomitant treatment. As mentioned earlier, some of the studies use more rigorous inclusion criteria. The German study and Frankel’s analysis of the tibia nonunions required a four-month interval between the last surgery and the start of treatment while Heppenstall required three months. The German, French, and Dutch studies as well as the Frankel and Heppenstall studies required radiographic assessment of the fracture to ensure nonunion. An unpublished retrospective analysis of the registry data by Heppenstall et al. separated the US data into two groups based on expanded inclusion criteria (9 month fracture age, 3 months since surgical intervention). The patients that met the 3 month post-surgery requirement, in addition to a 9 month fracture age, were designated as the core group and showed an 80% healed rate. Using the patients as his/her own control, a comparison of core group heal rates was statistically significant (p=0.00001). The most recent report of the registry data is in unpublished data provided by Exogen that looks only at patients that have completed treatment with the ultrasound device. This review of all the registry data reports a nonunion (256 days post-fracture) heal rate of 83%. Overall, the studies and reviews found heal rates for nonunions between 80-100% (See Table 1). The intention-to-treat values ranged from 64-82%.8 Table 1: Nonunion Results
Another consideration with respect to study design centers on the applicability of the study procedures beyond the research setting. The majority of the patients studied in these studies were treated at home under the instruction of their own physician. Therefore, the studies conducted on the ultrasound treatment of nonunion are relevant to the everyday clinical setting. Given that the results are applicable in the clinical setting, one must then ask whether those same results applicable to the Medicare population. The healing process is slower in the older population, but age has not been proven an independent predictor of healing disorders. Rather, comorbidity is the issue, of which age is most likely a covariate. Other factors, such as diabetes, vascular insufficiency, or certain prescription medications are correlated with advanced age and can affect the rate of healing. Of note, four of the studies analyzed for this decision stratified heal rates by age and found no significant difference among age groups. The third main issue in analyzing the data is the clinical/functional relevance of the outcome measures. The studies reviewed all use a primary outcome measure of healed or failure to heal. Healed is generally defined by radiographic guidelines (3 of 4 cortices bridged for long bones or soft callus bridge of the fracture gap) and clinical assessment (no pain with gentle stress or weightbearing). The dichotomous outcome (healed/failure to heal) is the accepted measure of success of the treatment. As previously discussed, nonunions are, by definition, unable to heal without some type of intervention. In that case, an outcome of healed, in the absence of any concurrent therapy, can be attributed to the ultrasound therapy and would be clinically relevant. Many of the studies also use heal time, measured in days, as a second outcome measure. Heal time, without associated functional outcomes, has limited clinical relevance. Even if a study indicated that ultrasound therapy worked more quickly than alternative treatments, the functional benefits of earlier healing would have to be addressed. For example, do patients return to work sooner? Is time to weightbearing reduced? Are casts removed more rapidly? Is there a reduction in morbidity? The fourth issue to consider when analyzing the data is the effectiveness of ultrasound treatment for different types of bones. Because bones have diverse function, blood supply, and structure, the effects of the ultrasound stimulator may vary for different bones. Of the articles reviewed, three studies (Gebauer, Moyen, Heppenstall) statistically compared heal rates for long bones vs. other bones. Two of the three studies shows no difference in heal rates between long bones and other bones. Gebauer et al. reported a success rate of 90% for long bones versus 69% for other bones which was statistically significant (p=0.05). The authors attribute this difference to the fact that, of the non-long bones that failed, 60% were atrophic scaphoids over 10 years old. Four studies (Gebauer, Moyen, Heppenstall, Nolte) statistically compared heal rates across all individual bones that could have included short, long, flat and irregular bones. Three of the four studies showed no statistical difference. Again, the Gebauer analysis resulted in a statistically significant difference showing a heal rate of 33% in the scaphoid versus 100% in the clavicle (p=0.02). This difference most likely resulted from the atypical age and structure of the scaphoid nonunions. In the other studies reviewed in this analysis, scaphoid heal rates typically varied from 80-100% (See Table 2). An analysis of the registry data (See Table 3) reveals that other non-long bones have similar heal rates to long bones. Heal rates range from 72-94%, which are consistent with the results for long bones. Table 2: Heal Rates by Bone Type
Table 3: "Other" Bones from Registry
Conclusion In conclusion, HCFA’s analysis of the data suggests that ultrasound osteogenic bone stimulation is a reasonable and necessary treatment for established nonunions. Although the evidence and the study designs were minimally acceptable, the study results were consistent and applicable to the Medicare population. Additionally, the outcome measures were clinically and functionally relevant, and these outcomes were similar for all types of bones. The policy will cover nonunions for fractures of all bone types; however, the policy will limit the use of the ultrasound stimulator to patients that have failed one prior surgery for the fracture. The prior surgery requirement is based on the characteristics of the patient population studied in the international studies (Gebauer, Moyen, Nolte) in which nearly 80% of the patients had at least one prior surgical intervention. This decision, as well as the FDA approval, relies primarily on these foreign studies, which were more well-controlled and rigorous than the Exogen registry. Coverage for patients who cannot undergo surgery due to comorbidities, or who refuse surgery will be considered as more information becomes available. We encourage physicians, patients, and manufacturers to engage in further dialogue with HCFA concerning the study designs necessary to expand this coverage. If 80% of nonunions can be healed with the use of the ultrasound device, a prospective comparison to surgical intervention as a primary nonunion treatment would strengthen the evidence, and potentially demonstrate an important improvement in clinical care for patients with nonunions. As Medicare continues to implement a more evidence-based decision making process, the question of study design becomes increasingly important. The use of the registry as part of this coverage determination was based on the specific nature of nonunion and the relevant outcomes provided by the registry. This particular decision should not be viewed as an assertion that registries are generally acceptable forms of evidence on which to base coverage decisions. As stated in the document, there is no requirement that all technologies be supported by randomized, prospective, placebo-controlled studies; however, technologies should be supported by studies that utilize the best design possible, giving the most clinically useful and functional outcomes. Regardless of the fact that the registry was allowed as a substantive portion of the data in this case, better, prospective clinical trials could have been performed. The evaluation of technologies based on the most well-designed studies for a particular technology or disease will benefit both the device industry and Medicare beneficiaries.
Rescind the national noncoverage policy for Ultrasonic Osteogenic Stimulators. Amend CIM 35-48 to state: B. Ultrasonic Osteogenic Stimulators.--An ultrasonic osteogenic stimulator is a non-invasive device that emits low intensity, pulsed ultrasound. The ultrasound signal is applied to the skin surface at the fracture location via ultrasound, conductive, coupling gel in order to stimulate fracture healing. Ultrasonic osteogenic stimulators are covered as medically reasonable and necessary for the treatment of fractures only when the following conditions are met:
Nonunions of the skull, vertebrae, and those that are tumor-related are excluded from coverage. The ultrasonic osteogenic stimulator may not be used concurrently with other noninvasive osteogenic devices. The national noncoverage policy relating to ultrasonic osteogenic stimulators for fresh fractures and delayed unions remains in place. This policy relates only to nonunion as defined above. 1 See Decision Memorandum Electrical Stimulation for Fracture Healing, November 9, 1999, at http://www.hcfa.gov/quality/8b.htm 2 Exogen was recently acquired by Smith & Nephew of Memphis, Tennessee. 3 Physicians that prescribed the ultrasound device completed periodic documentation of their patient's demographics, case specifics, and outcome. The most recent published studies analyze patients through July 14, 1997. At that time, there were 6,535 fractures that had completed treatment. As of June 15, 2000, 10,056 fractures in the United States had completed treatment with ultrasound. 4 The other studies (Gebauer et al., Nolte et al., Moyen et al., and Choffie) involve German, Dutch, French, and Brazilian patients that are not included in Exogen's registry. 5 Questions remain, however, about the accuracy and completeness of the Exogen registry. 6 Of note, the FDA relied primarily on the Gebauer (German) and Nolte (Netherlands) studies for the PMA approval. 7 Authors conservatively chose 5% heal rate though they note that nonunions have essentially a zero probability of healing without intervention. Such an assumption underestimates any positive effect. 8 This is a modified intent-to-treat analysis, since this type of statistical testing is used only for randomized control trials.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

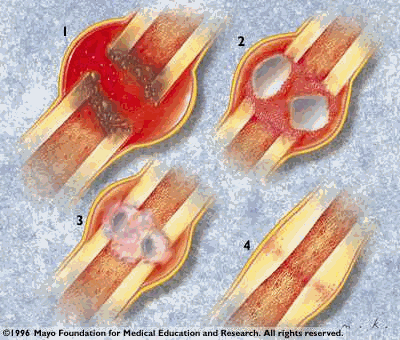 Inflammatory: The physical
disruption of the bone, blood vessels, and surrounding soft tissue
causes the formation of a hematoma (collection of blood in the
fracture site) and edema. Following tissue necrosis in the area,
macrophages and osteoclasts are recruited to remove the biological
debris. The stimulation and proliferation of osteoblasts (which
build bone) and endothelial cells (which line blood vessels) mark
the end of the inflammatory phase.
Inflammatory: The physical
disruption of the bone, blood vessels, and surrounding soft tissue
causes the formation of a hematoma (collection of blood in the
fracture site) and edema. Following tissue necrosis in the area,
macrophages and osteoclasts are recruited to remove the biological
debris. The stimulation and proliferation of osteoblasts (which
build bone) and endothelial cells (which line blood vessels) mark
the end of the inflammatory phase.