Top
of Report
Appendix 2: Detailed Description of MSRI Reduction Products
PROCESS: METHOCEL®
Background/Process Description
The METHOCEL® process is part of the Global Specialty Chemicals
business within Dow. There are three manufacturing sites including
the Midland site, Plaquemine, Louisiana and Stade, Germany.
METHOCEL® is Dow’s trade name for a line of methylcellulose and
hydroxypropyl methylcellulose water-soluble polymers. These products
are used for their water retention, viscosity modification and other
properties in a wide variety of markets including food,
pharmaceutical, ceramics, construction and other uses.
Cellulose from wood or cotton is used as a primary raw material
and is modified by reaction with sodium hydroxide, chloromethane and
propylene oxide. First, the cellulose is treated with the sodium
hydroxide to activate the cellulose as alkali cellulose. Then, the
chloromethane is used in an alkylation reaction to form the final
product.
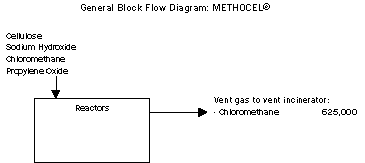
Sources and Baseline Conditions
The largest source of MSRI waste from the process is the vent gas
from the reactors. Chloromethane is the only MSRI chemical within
this waste stream and is generated as an unreacted raw material. The
vent gas also contains non-MSRI chemicals including dimethyl ether,
a byproduct formed in the chemical reaction.
Pollution Prevention Options and Reductions
There were no pollution prevention options implemented during the
course of MSRI.
Non Pollution Prevention Reductions
Three options were examined for the vent gas stream. 1) On-site
recovery of chloromethane followed by purification and sale of other
stream constituents. 2) On-site recovery of chloromethane followed
by shipment of other stream constituents to the Plaquemine site for
conversion to chloromethane; and 3) Off-site recovery of
chloromethane at the Plaquemine site followed by conversion of other
stream constituents to chloromethane. These three alternatives were
examined for technical risk, market risk, capital requirements and
economic value. After considering all factors, the business chose to
implement the off-site recovery and conversion option (3). This
waste-to-product option does not fit the MSRI definition of
pollution prevention and so is not counted toward the MSRI goals. It
is, however, an improvement to the current management option,
incineration.
Financial Savings Costs
The waste to product project is expected to reduce total wastes
by approximately 4.2 million pounds and have a payback period of
approximately 4 years.
PROCESS: ION EXCHANGE
Process Description and Sources
The Ion Exchange process is part of the Liquid Separations
business within Dow’s Global Specialty Chemical business. The Ion
Exchange process produces a range of DOWEX® anion and cation
exchange resins. These resins are used in various water treatment
applications for industrial and residential uses.
The primary raw materials in the ion exchange process are
methanol, formaldehyde, HCl, catalyst and a polymer, which is
produced at the Midland site in another process. The chemical
reaction in the ion exchange process creates a substituted polymer
with a functional group, which is used as an exchange site to
capture unwanted cations and anions from water as it passes through
the resin.
There are two primary sources of wastes in the process. The first
is vent gas from the reactors, which is burned in a vent gas
incinerator. Chloromethane, which is produced as a byproduct of the
chemical reaction, is the primary chemical within this waste stream.
The second source is generated in the recovery system and consists
of accumulated tars and raw materials that are not recovered. The
tars created in the reaction prevent the recovery of additional raw
materials and reducing tar formation was the focus of pollution
prevention investigations.
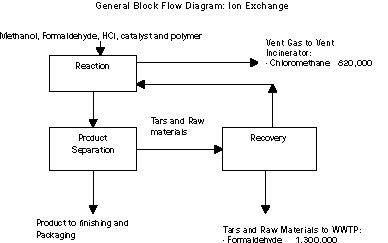
Pollution Prevention Options and Reductions
Several pollution prevention options were developed as part of
extensive studies of the basic chemistry of the reaction looking for
the underlying causes for waste generation.
Four options focused on reducing tar formation and increasing the
recovery of raw materials. The first option reduced the solvent
content of the polymer used as a raw material feedstock in the
process. Dow discovered that reducing residual solvent in the
polymer reduced the generation of tar and allowed more raw materials
to be recovered from the tar for reuse. The second option changed
catalyst use within the reaction, which also resulted in less tar
and increased recovery. The third option changed reaction
conditions, increasing yield and thus sending fewer raw materials to
the recovery system. With these changes in waste generation within
the reaction, Dow was then able to implement the fourth option,
which involved changing the operation of the raw material recovery
system to increase recovery. When fully implemented, these four
options will almost completely eliminate the loss of raw materials
to the wastewater treatment plant.
In addition to the options to reduce tars and improve raw
material recovery, Dow also modified reaction conditions to reduce
the formation of Chloromethane vented from reactors to the vent
incinerator.
| Source |
Chemical |
Estimated Release Reduction
(pounds) |
Estimated Waste Reduction
(pounds) |
| Vent gas to vent incinerator |
Chloromethane |
798 |
800,000 |
| Tars and Raw Materials to WWTP |
Formaldehyde |
63,621 |
1,300,000 |
Financial Savings Costs
Equipment changes needed to decrease
solvent in the polymer and change reaction conditions cost
approximately $330,000. Other changes detailed above did not require
capital. The investment will reduce the cost of lost raw materials
and waste treatment by approximately $3.3 million per year.
| Source/project |
Quantity (lbs) |
Lost Raw Material
Savings/Year |
Waste Treatment
Savings/Year |
Project Cost |
| Vent gas to vent incinerator |
800,000 |
$66,000 |
$62,000 |
$330,000 |
| Tars and Raw Materials to WWTP |
4,900,000 |
$1,400,000 |
$1,800,000 |
|
PROCESS: STYRENE-BUTADIENE LATEX (SB LATEX)
Process Description and Sources
The Styrene-Butadiene Latex (SB Latex) process is part of Dow’s
Global Emulsion Polymers Business. The SB Latex product is used in
coatings for coated paper and paperboard. It is also used in
adhesives in the carpet industry and as binders in a variety of
other applications.
Styrene and butadiene are the primary raw materials that are used
in the presence of an initiator to produce emulsion polymers. During
the reaction process, a fraction of the raw materials are converted
into finished products. The uncon- verted monomers are sent to a
recovery system with any excess disposed of by incineration.
An impurity in the butadiene, a "dimer" which is formed when
butadiene reacts with itself, plays a critical roll in waste
generation and its presence dictates what quantities of styrene and
butadiene are generated as waste. As the dimer builds up in the
recovery system, it must be periodically purged for incineration at
the on-site RCRA incinerator.
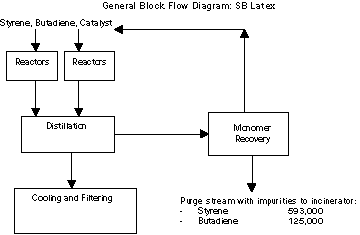
Pollution Prevention Options and Reductions
Two pollution prevention options are being pursued: modified
recovery techniques, and reducing dimer levels in purchased
butadiene. The first option, which was implemented during the MSRI
timeframe, modifies Dow’s production schedules and improves the
method for recovering raw materials. In combination, these
techniques reduce the quantity of the purge stream sent to the
incinerator. Dow rearranged existing handling systems to make this
possible.
The second pollution prevention option focuses on reducing dimer
levels in the incoming butadiene. Dimer formation is directly
related to time and temperature. The higher the temperature and the
longer the storage time the more dimer is formed. Dow conducted
extensive analysis of the dimer levels "as shipped" by their
suppliers, "as received" at the plant, transit time, and storage
temperatures. Dow developed a model to predict dimer levels. From
these studies, Dow believes additional reductions of 300,000 to
400,000 pounds of MSRI waste can be achieved if their suppliers load
butadiene at lower temperatures. Dow has begun discussions with its
principal butadiene supplier to discuss reducing the temperature of
butadiene at the time of loading. Almost all users of butadiene, no
matter what the product they make or in what country they are
operating, have wastes that are a consequence of dimer in butadiene.
Dow will attempt to convince its supplier that a competitive
advantage can be gained by supplying its customer with lower
dimer-content butadiene by loading colder.
| Source |
Chemical |
Estimated Release Reduction
(pounds) |
Estimated Waste Reduction
(pounds) |
| Purge stream from
recovery system |
Styrene |
0 |
208,000 |
| Butadiene |
0 |
44,000 |
Financial Savings Costs
Pollution prevention techniques
reduced total wastes by approximately 30 %, saving $105,000 per year
in lost raw materials and waste treatment costs.
| Source |
Quantity reduced (lbs.) |
Lost Raw Material
Savings/year |
Waste Treatment
Savings/Year |
Project Cost |
| Purge stream from recovery system |
252,000 |
$80,000 |
$25,000 |
$
50,000 |
The capital needed to modify the current process to achieve these
results amounted to $50,000. However, this expenditure reflects the
fact that Dow had an existing handling system and other equipment in
place at the Midland site. If this equipment had not been available,
the project would have cost an estimated $500,000. At this level,
the project may not have been funded. Dow is not pursuing this
option at other sites at this time because it does not have the
necessary spare equipment. However, the cold loading agreement is
being pursued with suppliers. If suppliers load butadiene into rail
cars at lower temperatures, styrene and butadiene waste will be
reduced at both Midland and other Dow SB Latex plants in North
America.
PROCESS: BULK PHARMACEUTICAL (PHARMA)
Process Description and Sources
Dow produces pharmaceutical products under contract for another
company. These products are part of the Contract Manufacturing
Services unit of Dow’s Global Specialty Chemicals business. The
federal Food and Drug Administration (FDA) approves the products and
production processes used in manufacturing.
In the process examined for MSRI, methylene chloride is used as a
chemical processing aid to keep raw materials and intermediates in
solution as they react to form the final product. Methylene chloride
is first distilled during the product reaction and then again during
the quench reaction. The methylene chloride is then burned in the
on-site RCRA incinerators.
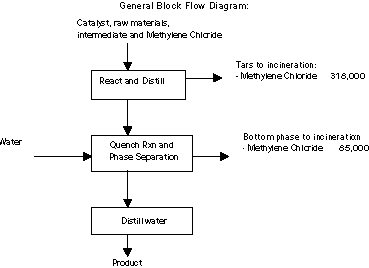
Pollution Prevention Options and Reductions
Because of the difficulty of qualifying a new process through the
Food and Drug Administration (FDA), a change of solvents was not
examined. This led Dow to consider in-process recycling options.
Process changes to incorporate these options required approval from
the FDA, which is sometimes cited as a disincentive for making
pollution prevention changes in the pharmaceutical industry. Of
particular concern was the potential build up of impurities in the
system and the impact of these impurities, if present, on the
product. However, since Dow had included an option for in-process
recycling in its original FDA application, approval could be
expedited.
Methylene chloride from the quench reaction separation step
contains water. Water would complicate the operation of a recovery
system and Dow opted not to explore options for this step first. The
pollution prevention option pursued was recovering the methylene
chloride from the product reaction distillation step. Dow conducted
pilot trials to determine any impacts on product quality and to
develop technical data for the design of the final system. To
conduct the trials, Dow installed temporary piping systems to
recycle the methylene chloride to produce a sufficient number of
batches. The product was tested in Dow’s quality assurance labs to
ensure no impurities were present.
| Source |
Chemical |
Estimated Release Reduction
(pounds) |
Estimated Waste Reduction
(pounds) |
| Distillation tars |
Methylene Chloride |
0 |
273,000 |
Initially, Dow recycled approximately 50 % of the methylene
chloride for quality assurance testing. Subsequently the number of
times solvent is recycled before incineration has slowly been
extended. Test results show no impact on product quality to date,
and Dow’s goal is to recycle about 95% of all of the methylene
chloride from the product reaction distillation step. This will
result in in-process recycling of 75% of the total methylene
chloride waste from the process. After the recycling of distillation
wastes has been fully implemented, Dow plans to begin work on the
quench reaction distillation waste. Both quench reaction
distillation improvements and in-process recycling will be pursued.
Financial Savings Costs
The construction of a permanent recovery system is estimated to
cost approximately $140,000 and save approximately $ 450,000 per
year in raw material costs and waste treatment costs.
| Source |
Quantity reduced (lbs.) |
Lost Raw Material
Savings/Year |
Waste Treatment
Savings/Year |
Project Cost |
| Distillation tars |
273,000 |
$ 350,000 |
$100,000 |
$
140,000 |
PROCESS: DURSBAN* (CHLORPYRIFOS)
Process Description and Sources
Dursban*, or chlorpyrifos, is a pesticide manufactured at the
Midland site by Dow AgroSciences LLC. Dursban*, an organophosphate
pesticide, is the most widely used insecticide on Earth. It is used
in a variety of applications such as combating termites, fleas and
other household pests as well as agricultural uses.
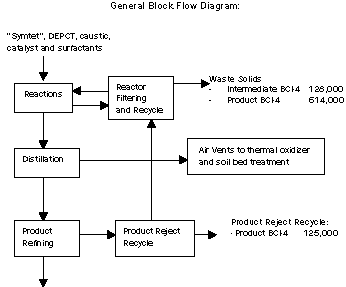
The process uses two primary raw materials: "symtet" (symmetrical
tetrachloropyridine) and "DEPCT" (diethyl phosphorochloridothioate)
along with sodium hydroxide, surfactants and catalysts. First,
sodium hydroxide is reacted with symtet to form an intermediate.
This intermediate is then reacted with DEPCT to form the final
product. The reaction mixture containing the final product then goes
through a series of separation and refining steps to isolate the
final product.
In the reaction, byproducts containing sulfur are created.
Because humans can detect organic sulfur compounds at extremely low
levels, these byproducts are vented to air controls including a
thermal oxidizer and a "soil bed" of bacteria that metabolize sulfur
compounds. The process also generates solids and sludge containing
intermediates and final product as waste. Pollution prevention
investigations focused on recovering product and an intermediate
from these wastes.
Pollution Prevention Options and Reductions
Dursban staff developed several pollution prevention options,
which can be grouped into three areas. The first option focused on
recovering and reusing the product recycle reject. This waste stream
is now in-process recycled back through the purification system to
recover the remaining product. The second group of pollution
prevention options included a series of small process changes such
as improved monitoring of process conditions and surfactant usage.
Finally, changes to the reactors were made to improve the yield of
product, decreasing the formation of unwanted byproducts.
| Source |
Chemical |
Estimated Release Reduction
(pounds) |
Estimated Waste Reduction
(pounds) |
| Product Recycle Reject |
Final Product BCl-4 |
0 |
125,000 |
| Process
changes |
Final Product BCl-4 |
0 |
162,000 |
| Intermediate product BCl-4 |
0 |
12,000 |
| Guard
filters/reactor yield |
Final Product BCl-4 |
0 |
32,000 |
| Intermediate product BCl-4 |
0 |
19,000 |
Financial Savings Costs
The savings from lost raw materials and avoided waste treatment
costs totaled approximately $157,000. It cost approximately $43,000
to implement these projects.
| Source |
Quantity reduced (lbs.) |
Lost Raw Material
Savings/year |
Waste Treatment
Savings/Year |
Project Cost |
| Product Recycle Reject |
125,000 |
$45,000 |
$12,000 |
$20,000 |
| Process changes |
174,000 |
$63,000 |
$17,000 |
Procedure changes |
| Guard filters/reactor yield |
51,000 |
$15,000 |
$5,000 |
$23,000 |
Another pollution prevention project was still in the design
phase in April 1999. This project would recover finished product by
adding new distillation and filtration equipment. Capital needed is
estimated to be $300,000. An additional 100,000 pounds of finished
product, which is now wasted, would be recovered. If these estimates
are correct, the project will be implemented. However, because
capital was not approved by April 1999, this potential reduction of
an MSRI chemical has not been counted toward the project goal.
* Trademark of Dow AgroSciences
PROCESS: DICHLOROPHENOXY ACETIC ACID (2,4D)
Process Description and Sources
Dow AgroSciences LLC produces 2,4-Dichlorophenoxy Acetic Acid
(2,4D), a member of the phenoxy family of herbicides. 2,4D is used
for the control of broadleaf weeds in grain and other crops. It is
also used on rangelands and for residential lawn and garden
applications.
The primary raw materials used in the process are
monochloroacetic acid and 2,4-dichlorophenol, both of which are
produced at the Midland site. (See process descriptions for CAC and
2,4 dichlorophenol.) Sodium hydroxide, hydrochloric acid and
tetrachloroethylene are also used in the process.
Monochloroacetic acid, 2,4-dichlorophenol and sodium hydroxide
are batch reacted to produce sodium 2,4-dichlorophenoxyacetate. The
reaction mixture is extracted with tetrachloroethylene to remove and
recover unreacted 2,4-dichlorophenol. The tetrachloroethylene is
then distilled for reuse. A portion of the tetrachloroethylene is
carried forward where it is stripped from the product mixture for
recovery. Hydrochloric acid is then added to convert the sodium salt
of 2,4D to the acid form, the final product.
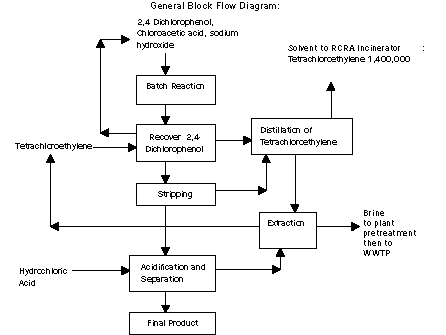
The largest source of MSRI waste in the 2,4D process is
tetrachloroethylene from the 2,4-dichlorophenol-recovery process.
This waste is burned in an on-site RCRA incinerator. Most vents from
the reaction step are treated in a scrubber and sent to an
incinerator. There are also fugitive emissions of
tetrachloroethylene and HCl from numerous seals, pipes, flanges,
pumps and other devices.
Pollution Prevention Options and Reductions
Pollution prevention options focused on reducing the
tetrachloroethylene waste generated in the recovery step. Dow found
a way to do a better job of reducing the formation of and breaking a
difficult emulsion formed in this step. As a result more
tetrachloroethylene is recovered. During 1999 and 2000 Dow will
continue to investigate additional ways to reduce
tetrachloroethylene losses from the recovery, although reductions
will not be counted toward the MSRI goal because funding was not in
place by April 1999.
| Source |
Chemical |
Estimated Release Reduction
(pounds) |
Estimated Waste Reduction
(pounds) |
| Distillation tars |
Tetrachloroethylene |
0 |
984,000 |
Citizen and NGO participants of the MSRI team expressed concern
over the timing and the manner in which this pollution prevention
option was developed. Dow developed the option early in the MSRI
project before Dow Elanco (the predecessor of Dow AgroSciences) had
officially agreed to participate, but after MSRI’s pollution
prevention consultant had begun a dialog with the plant. The idea
was reported to the group "after the fact" and did not follow the
participatory process used for other processes. In a more fully
participatory process, citizen and NGO participants would likely
have asked 2,4D staff more fundamental questions about the use and
generation of toxic substances. These questions may have led to
different options being considered. For example, why was
tetrachloroethylene used as the solvent? Are substitutes available?
In the past, Dursban was able to change process chemistry
eliminating the use of methylene chloride in a new water-based
process. Are similar opportunities available for 2,4D?
Tetrachloroethylene is used to recover unreacted raw material. Can
the yield of the process be improved to reduce the quantity of
unreacted raw material? These kinds of questions were pursued with
other businesses, but not with 2,4D.
Financial Savings Costs
The emulsion project cost approximately $140,000 and resulted in
a total saving of approximately $525,000 annually.
| Source |
Quantity reduced (lbs.) |
Lost Raw Material
Savings/year |
Waste Treatment
Savings/Year |
Project Cost |
| Distillation tars |
984,000 |
$215,000 |
$310,700 |
$140,000 |
PROCESS: CHLOROPHENOL (2,4-DICHLOROPHENOL)
Process Description and Sources
Dow AgroSciences LLC produces 2,4-dichlorophenol (DCP) at the
Midland site. All of the DCP produced is used on-site as a raw
material for the manufacture of 2,4D.
The process uses phenol and chlorine as raw materials. The
chlorination reaction produces DCP and byproduct hydrochloric acid,
which is used in other processes on-site. The DCP is then purified
by distillation prior to storage.
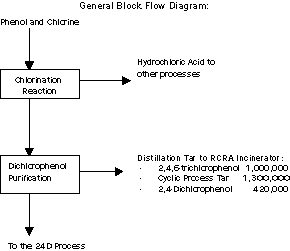
The chlorination reaction also creates byproducts, which are the
principal wastes generated in the process. One byproduct is
2,4,6-trichlorophenol, which is generated by the addition of a
third, unwanted chlorine to the phenol molecule. Another chlorinated
waste stream, identified for MSRI purposes as Cyclic Process Tar
BCl-2, is generated by the addition of chlorine to other unintended
sites on the phenol molecule. These wastes are separated from DCP by
distillation and burned in an on-site RCRA incinerator. Some DCP
distills over with these wastes and is also incinerated.
Pollution Prevention Options and Reductions
No pollution prevention options were developed during the course
of MSRI. A working relationship between the plant’s improvement
leader and the independent pollution prevention assessor was not
established until December 1998.
DCP process staff will focus pollution prevention efforts in 1999
and 2000 on improving the "selectivity" of the chlorination
reaction. This would improve the yield of 2,4 dichlorophenol
production and decrease formation of the 2,4,6-trichlorophenol and
other unintended byproducts. A study of the kinetics of chlorine
addition indicates that a reduction of approximately 40 % in 2,4,6
TCP and Cyclic Process Tar BCl-2 wastes is possible. Another future
pollution prevention option that will be considered is improved
separation of the product in the distillation step. A 50 % recovery
of DCP from the waste is possible. Dow will continue to pursue these
options, although reductions will not be counted toward the MSRI
goal because funding was not in place by April 1999.
PROCESS: CHLORO ACETYL CHLORIDE (CAC)
Process Description and Sources
The Chloro Acetyl Chloride (CAC) process is part of the Global
Chlorinated Organics business within Dow. The Midland site is Dow’s
only operating CAC plant. CAC is used primarily as an intermediate
in the manufacture of monochloroacetic acid (MCAA). Most of the MCAA
is sold. However, some is used as an intermediate at Midland to
manufacture the pesticide 2,4 Dichlorophenoxy acetic acid, more
commonly known as 24D.
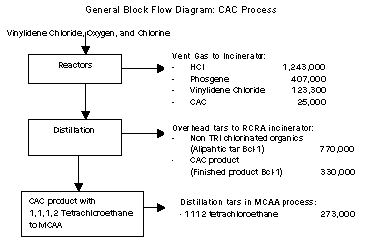
Vinylidene Chloride and oxygen are the primary raw materials used
to produce CAC. The oxidation reaction is highly exothermic,
generating significant heat. Currently, the reactor is cooled with
water from a cooling tower system.
The source of all wastes in CAC is the formation of byproducts in
the reactor. These wastes exit the process at two primary sources:
vent gases from the reactors and overhead tars from distillation.
Waste from these two sources account for about 18 % of all MSRI
wastes.
Pollution Prevention Options and Reductions
Several pollution prevention options were explored including
improved cooling, distillation column optimization and in-process
recycling. The pollution prevention option of choice focused on the
fundamentals of the chemical reaction to improve the overall
efficiency through improved cooling. All the wastes from the process
are generated as byproducts through inefficiencies in the reaction.
Lower reaction temperature would decrease the formation of these
unintended byproducts and increase yield of CAC product. Dow
determined that additional cooling could be provided through
refrigeration instead of the existing cooling towers. Improved
refrigeration was estimated to reduce the generation of byproduct
wastes by approximately 1.8 million pounds, 1.3 million of which is
MSRI chemicals.
| Source |
MSRI Chemical |
Estimated Release Reduction
(pounds) |
Estimated Waste Reduction
(pounds) |
| Vent Gas to
Incinerator |
HCl |
0 |
480,000 |
| Phosgene |
0 |
156,000 |
| Vinylidene Chloride |
0 |
48,000 |
| Overhead
Tars |
Non-TRI chlorinated |
| Organics (aliphatic tar BCl-1) |
0 |
500,000 |
| CAC (finished product BCl-1) |
0 |
10,000 |
| MCAA distillation tars |
1,1,1,2tetrachloroethane |
0 |
120,000 |
Financial Savings Costs
The avoided cost of lost raw materials and waste treatment from
CAC was estimated to be $475,000 per year.
| Source |
Quantity (lbs.) |
Lost Raw Material
Savings/Year |
Waste Treatment
Savings/Year |
Project Cost |
| Reaction |
1,800,000 |
$ 225,000 |
$250,000 |
$300,000 |
Initially, Dow prepared a "Project Definition," a package of
technical and financial information used in Dow’s internal funding
approval process, based on purchasing new refrigeration equipment.
New equipment was estimated to cost $500,000. In addition, $20,000
was requested so technical staff could conduct more detailed
technical analysis for the project. At this early stage in the
funding approval process, the project was considered only marginally
attractive. . However, it was clear that if Dow followed this
"typical" project development and approval process, it would be a
challenge to obtain funding approval before the end of April.
In an effort to improve the economics of the project, Dow CAC
staff contacted brokers of used refrigeration equipment. A suitable
refrigeration unit was identified which improved the Discounted Cash
Flow from 25 % to 48 %. CAC staff then contacted the business
leaders who make funding decisions to discuss the new information.
Funding was approved in less than one week. A search is now underway
for idle refrigeration equipment within Dow which, if found, will
improve project economics even further. The project will also
increase production capacity. However, financial benefits from
increased production were not part of the economic justification for
the project because Dow evaluation rules require the increased
production to be pre-sold before its value can be counted.
Detailed technical review of the equipment is expected to be
complete by mid summer and installed and operational by April 2000.
PROCESS: ETHOCEL®
Process Description and Sources
The ETHOCEL® process is part of Dow’s Global Specialty Chemicals
business. The manufacturing plant has been in operation at the
Midland site for over 60 years. ETHOCEL® is used in pharmaceutical
applications.
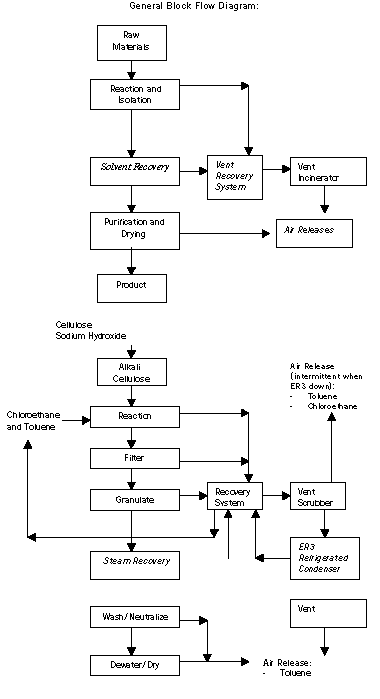
Air releases of toluene and chloroethane are the largest source
of MSRI waste from the process. Vents from the reaction and recovery
vessels are collected and passed through a refrigerated toluene
scrubber. Any solvent not recovered by the time product leaves the
solvent recovery step is lost as vent releases during the washing
and drying steps.
Pollution Prevention Options and Reductions
Two pollution prevention options were implemented. The first
included constructing a solvent recovery unit that uses steam to
remove organic compounds from the product stream as it leaves the
reaction train. The second pollution prevention project was the
installation of another refrigerated condenser for recovery of
toluene and chloroethane. This system was attached to the vent of
the existing chilled toluene recovery system. The new refrigerated
condenser was then connected to an existing on-site vent gas
incinerator because one of the reaction byproducts, ethylene, cannot
be condensed without expending a large amount of energy.
| Source |
Chemical |
Estimated Release Reduction
(pounds) |
Estimated Waste Reduction
(pounds) |
| Vent losses from
reaction and recovery |
Toluene |
243,000 |
243,000 |
| Chloroethane |
114,000 |
114,000 |
Financial Savings Costs
At $1.8 million, these were the highest cost projects in MSRI.
They accounted for almost 60% of the total expenditure of $3
million. The raw materials recovered are inexpensive, so the savings
gained by these investments were small. However, ETHOCEL® was by far
the largest emitter of TRI chemicals at the Midland site. The
initial driving force behind implementation of the projects was the
magnitude of the TRI chemical releases relative to the corporate
environmental philosophy However, the MSRI effort provided the
impetus for a concerted effort to focus on the quick start-up and
opimization of these unit operations.
| Source |
Quantity (lbs.) |
Lost Raw Material
Savings/Year |
Waste Treatment
Savings/Year |
Project Cost |
| Vent losses from reaction and
recovery |
357,000 |
$54,000 |
$0 (formerly released) |
$1,785,000 |
Non-Pollution Prevention Reductions
Ethylene and a small amount of entrained raw materials were
routed to an existing on-site incinerator. Incinerating these
materials will reduce net releases by an additional 269,000 pounds
per year: 30,000 pounds of MSRI chemicals (28,000 chloroethane and
2,000 toluene) and 239,000 pounds of non-MSRI chemicals (233,000
ethylene). The incineration of these materials, and the 9,000 pounds
incinerated by engineering plastics, are the only treatment
associated with the MSRI project. Materials incinerated are not
counted in MSRI release reductions.
PROCESS: SARAN*
Process Description and Sources
The SARAN* process is part of Dow’s Global Polyethylene business.
There are three general types of SARAN* resins produced at the
Midland site, each having vinylidene chloride (1,1-dichloroethylene,
or VDC) as a principal raw material. SARAN* products are used for
their barrier protection properties that prevent transmission of
gases, water and odors in various food packaging and other
applications.
While there are differences between the three processes, a single
general description is used for the process. The first step in the
process involves purification and storage of VDC monomer. Next, the
other raw materials are added to initiate the polymerization
reaction. The reaction mixture is then stripped to remove unreacted
raw materials. A portion of these materials are recovered and
reused. After stripping, the resin slurry is dewatered and dried
prior to storage and packaging of the product.
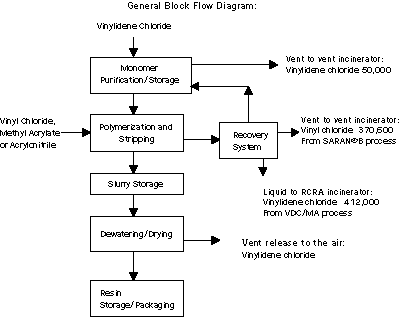
There are four primary sources of MSRI waste within the SARAN*
processes. The first source is a vent from the raw material storage
tanks connected to a vent incinerator. This waste is the result of
vapor losses during filling and transfer of vinylidene chloride. The
second source is also a vent to the vent incinerator. This loss is
from the recovery of unreacted monomers in the SARAN*B process and
consists primarily of vinyl chloride monomer. The third source is a
liquid waste generated in the recovery process for the VDC/MA
copolymer. This waste is burned in the on-site RCRA incinerator. The
last source is a vent release to the air from the slurry storage and
drying steps.
Pollution Prevention Options and Reductions
One pollution prevention option was implemented in this process.
The change reduced vent losses from the raw material storage step by
instituting new vapor balance procedures for transfers between
tanks. Previously, Dow transferred raw material once per day to one
of two tanks. Following the new procedures, there are two transfers
per day and the tanks are not filled completely. This allows vapors
displaced during filling to be vented into the headspace of the
other tank instead of vented to the incinerator.
Dow studied recovery options for the vinyl chloride monomer vent
losses from the recovery system in the SARAN*B process. Options
considered for recovery include Pressure Swing Adsorption (PSA),
increased pressure/condensation, and membrane separation. Dow has
budgeted $50,000 to pilot test new technologies during the summer of
1999. The focus of these tests is on the use of PSA. Expecting that
these tests will prove recovery is feasible, Dow has budgeted
capital for implementation in 2000. Since the commitment of capital
is dependent on tests to be conducted after the April 1999 deadline,
these reductions are not counted. PSA tests run on similar streams
at other Dow sites indicate that a 95% recovery rate of vinyl
chloride can be expected.
Dow also looked at options for reducing the liquid waste from the
VDC/MA process. Options considered for reducing this stream included
in-process recycling and improving yield through changes in reaction
chemistry. Concerns about the effect of MA recycle on product
quality make it likely that the focus of future investigations will
be on changes in chemistry. There are no firm plans for pursuing
either of these options at this time.
| Source |
Chemical |
Estimated Release Reduction
(pounds) |
Estimated Waste Reduction
(pounds) |
| Vent from storage tanks to vent
incinerator |
Vinylidene Chloride |
0 |
34,000 |
Financial Savings Costs
The pollution prevention option in the storage step will save
$13,000 annually. Instituting the new procedures did not require any
capital expenditure.
| Source |
Quantity reduced (lbs.) |
Lost Raw Material
Savings/Year |
Waste Treatment
Savings/Year |
Project Cost |
| Vent from storage tanks to vent
incinerator |
34,000 |
$10,000 |
$3,000 |
Procedure change
only |
* A trademark of The Dow Chemical
Company
PROCESS: HIGH IMPACT POLYSTYRENE (HIPS)
Process Description and Sources
The high impact polystyrene (HIPS) process is part of Dow’s
Global Polystyrene Business. The process produces STYRON® resins
that are used in a variety of injection molding and extruded
applications. STYRON® HIPS is used to manufacture products such as
toys, appliance parts, packaging and furniture.
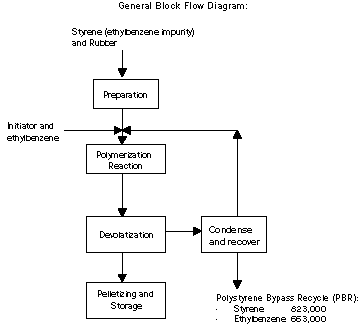
The process uses styrene and rubber as the primary raw materials.
Styrene is used as a reactant for the polymerization reaction and
rubber is added to increase strength and dent-resistance. In
addition to these primary raw materials, an initiator is needed to
start the polymerization reaction. This initiator is received in
liquid form diluted with ethylbenzene. Once the polymer is produced,
unreacted styrene and ethylbenzene remaining in the product is
removed through stripping and condensing. This styrene is then
reused in the process.
The primary by-product generated in the process is a purge stream
from the recovery and reuse of styrene. A portion of the material in
the recycle loop must be removed to prevent the buildup of
ethylbenzene, which is un-reactive, in the system. Styrene is lost
along with the ethylbenzene. The ethylbenzene comes from two
sources: as an impurity in the styrene and as the initiator diluent.
The by-product is sent to another Dow facility and is used as a raw
material to produce styrene.
Pollution Prevention Options and Reductions
A new concentrated form of the initiator was introduced. The
concentrated form contains much less ethylbenzene diluent. This
reduction in ethylbenzene inputs enabled Dow to reduce the amount of
purge from the recycle system. Lower purge volumes mean lower
styrene loss.
| Source |
Chemical |
Estimated Release Reduction
(pounds) |
Estimated Waste Reduction
(pounds) |
| Polystyrene Bypass
Recycle (PBR) |
Styrene |
0 |
456,000 |
| Ethylbenzene |
0 |
364,800 |
Financial Savings Costs
Equipment changes needed to use the concentrated catalyst cost
approximately $300,000. This investment saved approximately $270,000
per year in lost raw material cost.
| Source |
Quantity reduced (lbs.) |
Lost Raw Material
Savings/Year |
Waste Treatment
Savings/Year |
Project Cost |
| Polystyrene Bypass Recycle (PBR) |
820,800 |
$270,000 |
|
$300,000 |
PROCESS: ENGINEERING PLASTICS
Process Description and Sources
Three families of plastics are produced at the Midland site as
part of the Global Engineering Plastics business. These plastics
include TYRIL® (Styrene-Acrylonitrile or SAN), Mass ABS and Emulsion
ABS/SAN. These plastics can be formulated to give a wide range of
physical properties. Typical applications include housewares,
packaging, auto parts, appliances, and piping.
Styrene is the raw material common to each of these engineering
plastics. TYRIL® uses acrylonitrile to create a
styrene-acrylonitrile copolymer. Mass ABS and Emulsion ABS also add
polybutadiene rubber to enhance strength and other performance
properties.
The TRYIL® and Mass ABS processes use similar steps. Raw
materials react to form the polymer. The polymer is then stripped to
remove unreacted, volatile raw materials. Most of these raw
materials are recovered through condensation and returned to the
process. After stripping, the polymer is pelletized prior to storage
in large silos.
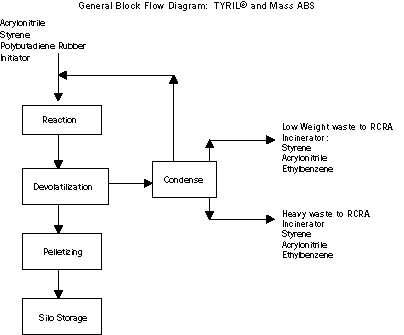
The largest sources of wastes from the process are generated in
the recovery of volatile raw materials. Two separate waste streams,
one of low molecular weight compounds and a second of higher
molecular weight compounds, are generated and sent to the RCRA
incinerator. A smaller source of waste is generated from the
equipment cleaning operations that use methylene chloride to clean
heat exchangers.
The Emulsion process uses different processing steps. Here, latex
ABS is produced. In addition to the latex ABS, a portion of the Mass
ABS and TYRIL® are used as intermediates to manufacture different
forms of the polymer.
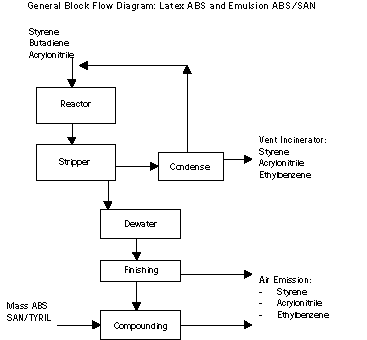
No liquid or solid MSRI wastes are generated in the recovery of
raw materials. Vent air losses are the primary losses from the
emulsion/latex process.
Pollution Prevention Options and Reductions
One pollution prevention option was developed in the Mass ABS
process. Methylene chloride was used to clean process equipment
(heat exchangers). Dow is replacing methylene chloride with a new
cleaning process. Currently, two options are being considered. One
option uses N-methyl pyrrolidone (NMP) for cleaning. Initial tests
results demonstrated that NMP is a better and less volatile cleaner.
In winter months, however, the cleaning tank will have to be heated.
The second option uses a non-TRI substance in a new cleaning process
currently being tested at another Dow site. Because six months
elapse before the heat exchangers are re-installed, both options
will be pursued during the summer of 1999. The NMP process works,
but the other process is attractive because it does not use a TRI
chemical (process uses heat and a high boiling point, recycled
petroleum solvent) and the cleaning would be done by one of Dow’s
contractors.
Dow is also pursuing an additional pollution prevention option
that was not fully developed within the 2-year time period for MSRI.
This option involves switching from a dry form of initiator to a
liquid form. Additional research and trial runs are needed and Dow
plans to continue these efforts.
| Source |
Chemical |
Estimated Release Reduction
(pounds) |
Estimated Waste Reduction
(pounds) |
| Cleaning solvent |
Methylene chloride |
339 |
10,000 |
Financial Savings Costs
The new cleaning process is expected cost approximately $1,000
and save approximately $1,000 per year.
| Source |
Quantity reduced (lbs.) |
Lost Raw Material
Savings/Year |
Waste Treatment
Savings/Year |
Project Cost |
| Heat exchanger cleaning |
10,000 |
$0 |
$1,000 |
$1,000 |
Non Pollution Prevention Reductions
During the MSRI project, Dow implemented additional treatment of
vent air losses. Dow installed additional collection and piping to
capture and send vent losses from the finishing step to an existing
vent incinerator. This additional treatment is expected to reduce
styrene vent releases by 9,000 pounds per year.






