| ||||

|
August 2000 Return to Aug 2000 contents page BRIEFING No more business as usual Although AIDS vaccines may still be years away, policy makers must act radically and swiftly to ensure global access to them, say two new analyses
EVEN if the scientific hurdles to developing AIDS vaccines can be overcome, low-income countries may still wait decades for access to those vaccines, warns a hard-hitting report (1) released last month. The report, from the International AIDS Vaccine Initiative (IAVI), concludes that unless there is a "monumental shift" in the world’s approach to the use of vaccines, millions of people will be needlessly infected with HIV while they wait for those vaccines to "trickle down" to them. The report calls for immediate and radical changes in the global approach to vaccine production, licensure, pricing, purchasing and distribution, and sets out a five-point action plan. Reality check The report comes soon after a separate analysis of the prospects for developing and using AIDS vaccines, from Josť Esparza of the WHO-UNAIDS HIV Vaccine Initiative and Natth Bhamarapravati of Mahidol University, Thailand (2). The authors urge that trials of vaccine candidates be stepped up and that plans for universal access be made now. "The ultimate irony would be that a vaccine developed in collaboration with less-developed countries could actually contribute to increasing the gap and inequalities that the AIDS pandemic has created," they say.
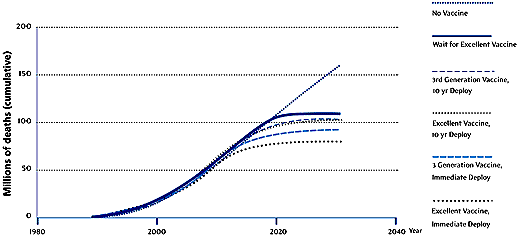
IAVI identifies key reasons for the slow introduction of existing vaccines into low-income countries. These include lack of money, the low priority placed on disease prevention by most governments, and, in some high-income countries, the political unpopularity of differential pricing policies for health products. In addition, manufacturers must navigate the "fragmented and uncoordinated" regulatory systems of different nations for approving vaccines, and must scale up production for global needs. In the case of AIDS vaccines, these problems are compounded, the IAVI report says, by additional challenges: crucially, in the poorer countries there is little or no infrastructure for distributing vaccines to the population groups that most urgently need immunizing against HIV — adolescents and sexually active adults. Most vaccines are given to infants and, although some have argued that HIV vaccines could also be given to this age group, the IAVI report says that such an approach could introduce further delays. The efficacy of a vaccine administered in infancy might not be known until many years of trials have passed, and the duration of protection would also be difficult to determine, says Widdus. "You could end up postponing [implementation] for 10 years and then still find that you need a booster in adolescence." On top of these problems, planning now for large-scale production is difficult because experimental AIDS vaccines are evolving fast. Moving target Whereas "first-generation" vaccines, as defined by IAVI, may provide only 40% protection and may require multiple doses, a "third-generation" vaccine might offer 90% protection, be administered orally, and require only occasional boosters. Clearly, each vaccine type would have its own specific requirements for volume, delivery and counselling. Overall, choices about the types of vaccines used and the speed at which they are introduced could decide the fate of millions of people over the course of the epidemic (Figure 1).
Source: IAVI Figure 1: Projected global AIDS
deaths with different vaccine strategies A third critical problem with HIV vaccines is that no one yet knows whether a vaccine based on one strain of the virus will protect against other strains. In many communities, particularly in Sub-Saharan Africa, multiple strains are now circulating. The report says that studies to establish whether vaccines can protect against several strains must be run in parallel and must be strategically coordinated. Otherwise the assessment process could take several additional years. IAVI lists five key requirements to ensure rapid access to vaccines:
Political leaders and the private sector are challenged to endorse the use of tiered pricing for AIDS vaccines, so that low-income countries will be able to pay what they can afford while manufacturers will still get a satisfactory return on their investment. The report calls for "credible" financial commitments from the industrialized nations to buy and deliver vaccines to developing countries. Much more effort is also needed, it says, to convince finance ministers and donors of the value of preventing disease, particularly AIDS which is almost always fatal and which affects young, productive adults. The report suggests that, on the basis of existing knowledge, an HIV vaccine could be cost-effective at prices up to 50 times higher than the traditional children’s vaccines. Detailed studies on the cost-effectiveness of hypothetical HIV vaccines have not been done yet. But the President of IAVI, Seth Berkley, says they are a priority.
"To think about intelligent healthcare packages takes time," says Widdus. "We need to start thinking about this now, not because there will be a vaccine next week, but because these things are intrinsically difficult and we are more likely to make mistakes if we rush at the last minute." The IAVI report’s fifth recommendation — that existing under-used vaccines against major diseases such as hepatitis B or Hib be rapidly and effectively introduced in developing countries through partnerships such as GAVI — will be the key test, it argues. If industry boardrooms are convinced that partnerships for the introduction of these vaccines can work, then partnerships for AIDS vaccines are also more likely to move ahead, says the report. Tore Godal, Executive Secretary of GAVI says: "We must not be paralysed by problems that are still hypothetical. Instead we should work hard to develop the vaccines themselves and then use every mechanism at our disposal — including GAVI — to get them quickly to those who need them most." References 1. AIDS Vaccines for the World: Preparing now to assure access. International AIDS Vaccine Initiative, July 2000. Download or read online summaries from http://www.iavi.org/ 2. Accelerating the development and future availability of HIV-1 vaccines: why, when, where and how? Josť Esparza and Natth Bhamarapravati. Lancet 355: 2061-66. Medline: www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10885368&dopt=Abstract
Return to August 2000 contents page
| |||||||||||||||
|
Contact GAVI | Guestbook | Text version | Credits and Copyright
| |||||||||||||||